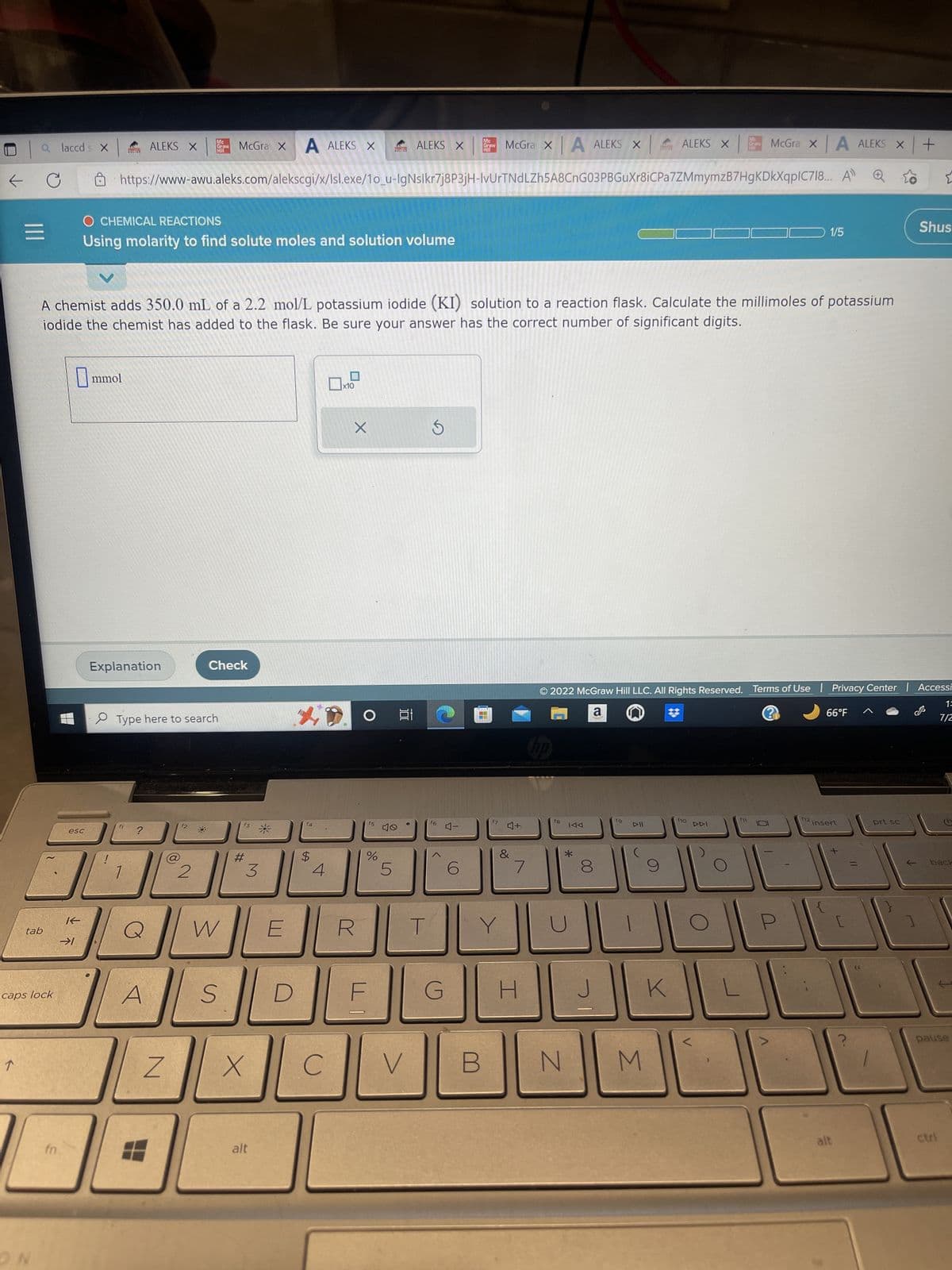
A chemist adds 350.0 mL of a 2.2 mol/L potassium iodide (KI) solution to a reaction flask. Calculate the millimoles of potassium iodide the chemist has added to the flask. Be sure your answer has the correct number of significant digits.
A chemist adds 350.0 mL of a 2.2 mol/L potassium iodide (KI) solution to a reaction flask. Calculate the millimoles of potassium iodide the chemist has added to the flask. Be sure your answer has the correct number of significant digits.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section: Chapter Questions
Problem 121IL: Suppose you dilute 25.0 mL of a 0.110 M solution of Na2CO3 to exactly 100.0 mL. You then take...
Related questions
Question
Answer must be correct significant di
Transcribed Image Text:a laccd s X
|||
tab
ON
caps lock
fn
esc
K-
→1
V
PARA
mmol
O CHEMICAL REACTIONS
Using molarity to find solute moles and solution volume
ALEKS X
Explanation
1
A chemist adds 350.0 mL of a 2.2 mol/L potassium iodide (KI) solution to a reaction flask. Calculate the millimoles of potassium
iodide the chemist has added to the flask. Be sure your answer has the correct number of significant digits.
?
Mc
Graw
KI
Type here to search
A
https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmymzB7HgKDkXqpIC718...
N
2
McGra X A ALEKS X
Check
W
S
#
3
X
alt
D
x
$
4
C
x10
Xx
O
10
%
ALEKS X
5
100
111
V
f6
G
4-
Mc
Graw
HI
6
B
McGra x A ALEKS X
f7
4+
&
7
H
18
*
N
8
© 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessi
a
@
J
fg
www
DII
3
ALEKS X
9
K
McGra XA ALEKS X +
fo
DDI
1/5
F12
66°F
insert
alt
Shus
prt sc
1:
7/2
back
pause
ctri
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning