A chemist carefully measures the amount of heat needed to raise the temperature of a 0.16 kg sample of C,H0, from 20.9 °C to 34.9 °C. The experiment shows that 3.53 × 10° J of heat are needed. What can the chemist report for the molar heat capacity of C,H100,? Be sure your answer has the correct number of significant digits. OJ- mol -1 -1 •K
A chemist carefully measures the amount of heat needed to raise the temperature of a 0.16 kg sample of C,H0, from 20.9 °C to 34.9 °C. The experiment shows that 3.53 × 10° J of heat are needed. What can the chemist report for the molar heat capacity of C,H100,? Be sure your answer has the correct number of significant digits. OJ- mol -1 -1 •K
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 12E: An aluminum kettle weighs 1.05 kg. (a) What is the heat capacity of the kettle? (b) How much heat is...
Related questions
Question
A chemist carefully measures the amount of heat needed to raise the temperature of a 0.16kg sample of C9H10O2 from 20.9°C to 34.9°C. The experiment shows that ×3.53103J of heat are needed. What can the chemist report for the molar heat capacity of C9H10O2? Be sure your answer has the correct number of significant digits.
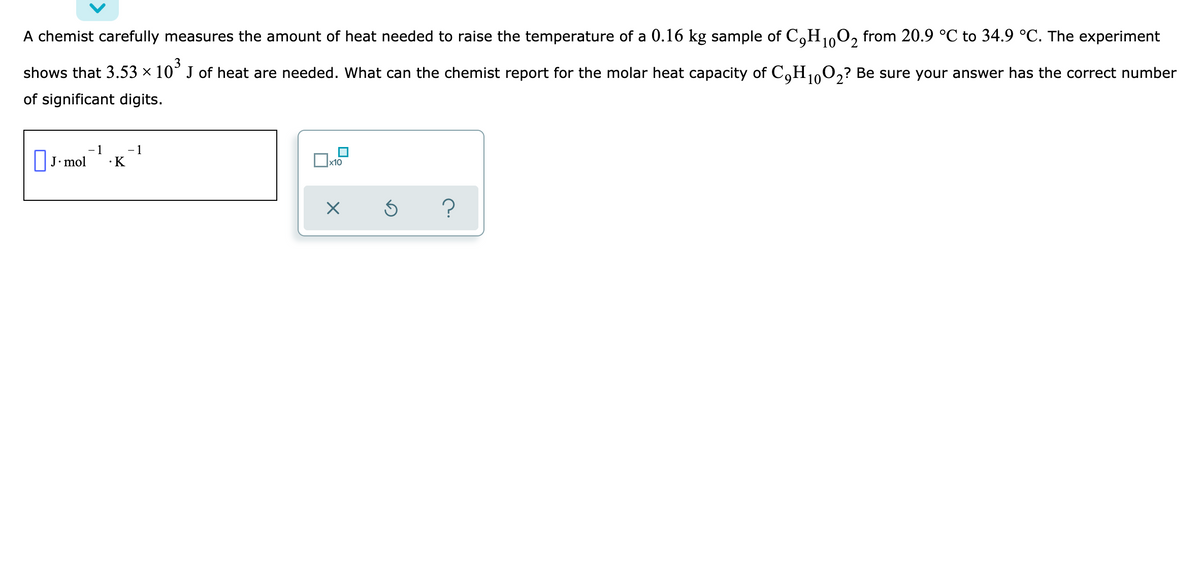
Transcribed Image Text:A chemist carefully measures the amount of heat needed to raise the temperature of a 0.16 kg sample of C,H0O, from 20.9 °C to 34.9 °C. The experiment
3
shows that 3.53 × 10° J of heat are needed. What can the chemist report for the molar heat capacity of C,H100,? Be sure your answer has the correct number
of significant digits.
|J. mol
1
- 1
•K
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning