A chemist has three different acid solutions. The first acid solu tion contains 15 % acid, the second contains 35 % and the third contains 65 % . They want to use all three solutions to obtain a mixture of 204 liters containing 25 % acid, using 2 times as much of the 65 % solution as the 35 % solution. How many liters of each solu tion should be used? The chemist should use liters of 15 % solution, liters of 35 % solution, and liters of 65 % solution.
A chemist has three different acid solutions. The first acid solu tion contains 15 % acid, the second contains 35 % and the third contains 65 % . They want to use all three solutions to obtain a mixture of 204 liters containing 25 % acid, using 2 times as much of the 65 % solution as the 35 % solution. How many liters of each solu tion should be used? The chemist should use liters of 15 % solution, liters of 35 % solution, and liters of 65 % solution.
Glencoe Algebra 1, Student Edition, 9780079039897, 0079039898, 2018
18th Edition
ISBN:9780079039897
Author:Carter
Publisher:Carter
Chapter6: Systems Of Linear Equations And Inequalities
Section6.1: Graphing Systems Of Equations
Problem 59PFA
Related questions
Question
100%
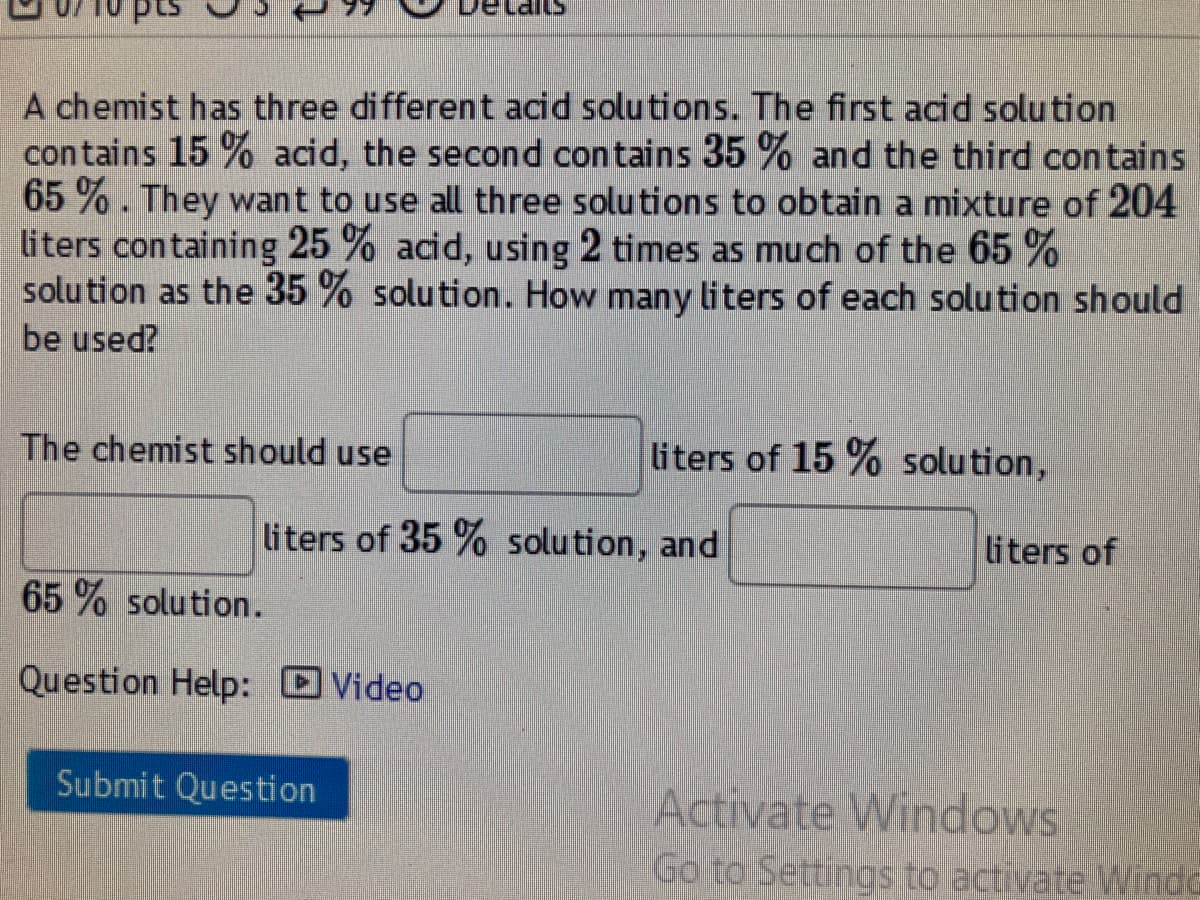
Transcribed Image Text:A chemist has three different acid solutions. The first acid solution
contains 15 % acid, the second contains 35 % and the third contains
65 % . They want to use all three solutions to obtain a mixture of 204
liters containing 25 % acid, using 2 times as much of the 65 %
solu tion as the 35 % solution. How many liters of each solution should
be used?
The chemist should use
liters of 15 % solution,
liters of 35 % solution, and
liters of
65 % solution.
Question Help: Video
Submit Question
Activate Windows
Go to Settings to activate Winda
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, algebra and related others by exploring similar questions and additional content below.Recommended textbooks for you

Glencoe Algebra 1, Student Edition, 9780079039897…
Algebra
ISBN:
9780079039897
Author:
Carter
Publisher:
McGraw Hill

Algebra for College Students
Algebra
ISBN:
9781285195780
Author:
Jerome E. Kaufmann, Karen L. Schwitters
Publisher:
Cengage Learning


Glencoe Algebra 1, Student Edition, 9780079039897…
Algebra
ISBN:
9780079039897
Author:
Carter
Publisher:
McGraw Hill

Algebra for College Students
Algebra
ISBN:
9781285195780
Author:
Jerome E. Kaufmann, Karen L. Schwitters
Publisher:
Cengage Learning


Elementary Algebra
Algebra
ISBN:
9780998625713
Author:
Lynn Marecek, MaryAnne Anthony-Smith
Publisher:
OpenStax - Rice University


Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage