A chemist uses 0 25 Lof 2.00 M H2S04 to completely neutralize a 2.00 Lof solution of NaOH The balanced chemical equation of the reaction is given below What is the concentration of NaOH that is used? O 0.063 M O 0 25 M O 0.50 M 1.0M
A chemist uses 0 25 Lof 2.00 M H2S04 to completely neutralize a 2.00 Lof solution of NaOH The balanced chemical equation of the reaction is given below What is the concentration of NaOH that is used? O 0.063 M O 0 25 M O 0.50 M 1.0M
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 151QRT: A mountain lake that is 4.0 km × 6.0 km with an average depth of 75 m has an H+(aq) concentration of...
Related questions
Question
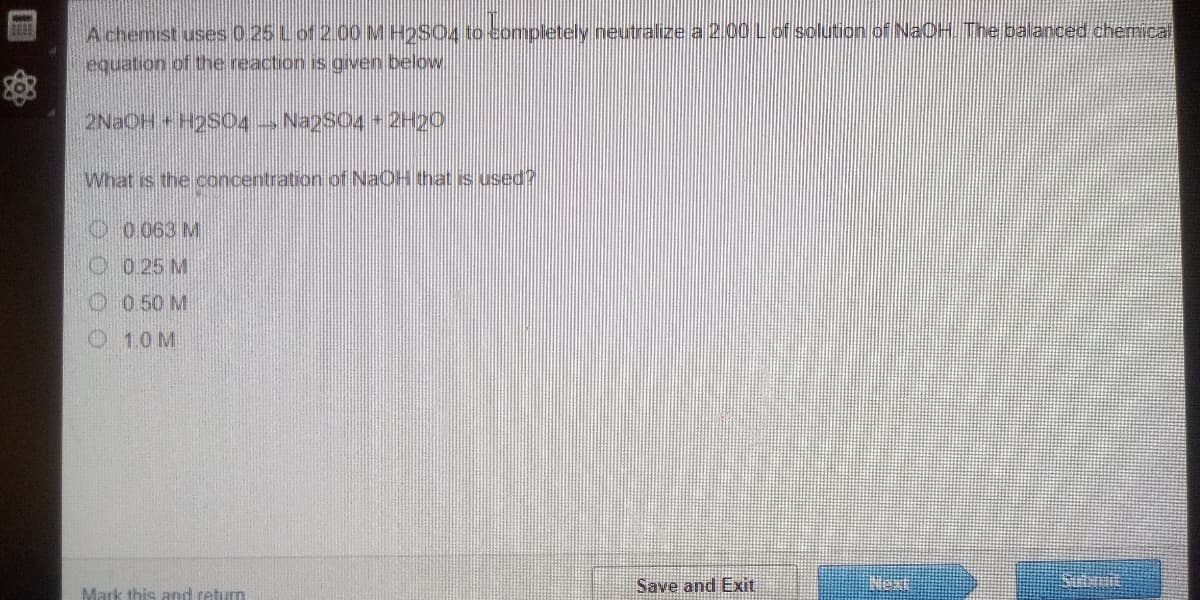
Transcribed Image Text:A chemist uses 0.25 Lof 2.00MH2SO4 to eompletely neutralize a 2.00 Lof solution of NaCH The balanced chemical
equation of the reaction is gven below.
2NAOH+H2S0O4 Naps04 2H00
What is the concentration of NaOH that is used?
O 0063 M
O 0 25 M
O 0 50 M
O 1.0M
Save and Exit
Next
Mark this and return
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning