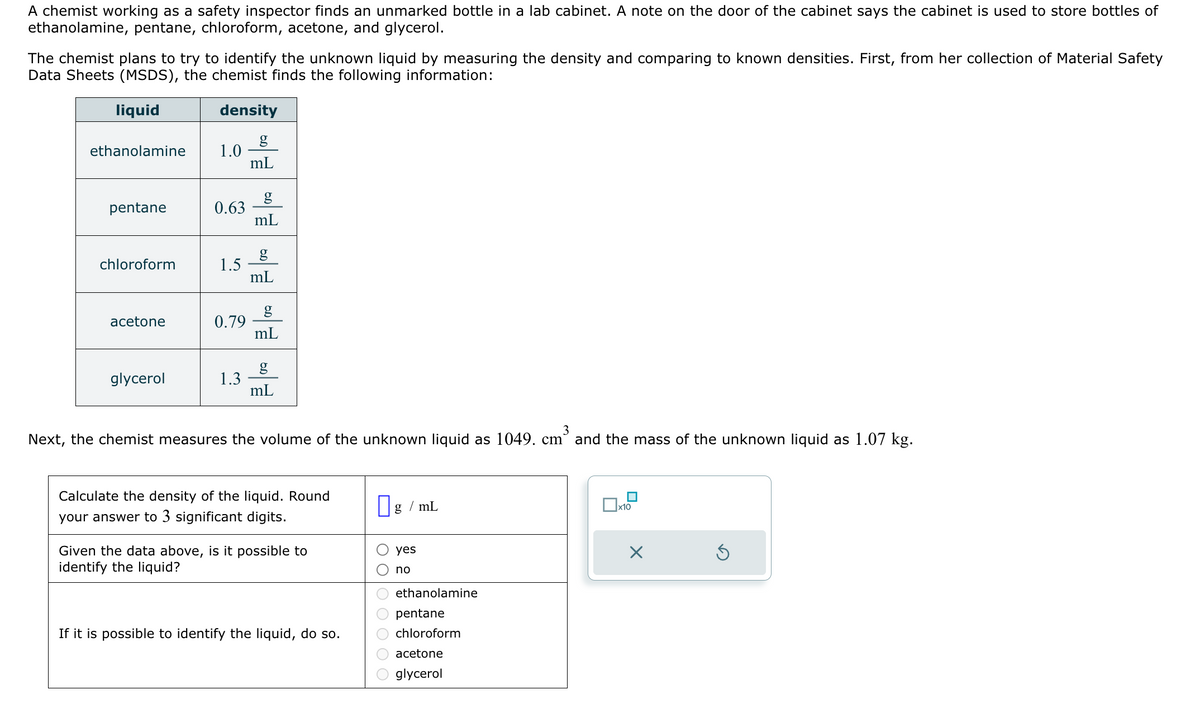
A chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of ethanolamine, pentane, chloroform, acetone, and glycerol. The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from her collection of Material Safety Data Sheets (MSDS), the chemist finds the following information: Next, the chemist measures the volume of the unknown liquid as 1049. cm and the mass of the unknown liquid as 1.07 kg.
A chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of ethanolamine, pentane, chloroform, acetone, and glycerol. The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from her collection of Material Safety Data Sheets (MSDS), the chemist finds the following information: Next, the chemist measures the volume of the unknown liquid as 1049. cm and the mass of the unknown liquid as 1.07 kg.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 5PS
Related questions
Question
A chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of ethanolamine, pentane, chloroform, acetone, and glycerol.
The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from her collection of Material Safety Data Sheets (MSDS), the chemist finds the following information:
Next, the chemist measures the volume of the unknown liquid as 1049. cm and the mass of the unknown liquid as 1.07 kg.
Transcribed Image Text:A chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of
ethanolamine, pentane, chloroform, acetone, and glycerol.
The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from her collection of Material Safety
Data Sheets (MSDS), the chemist finds the following information:
liquid
density
g
mL
ethanolamine 1.0
pentane
chloroform
acetone
glycerol
0.63
1.5
0.79
1.3
g
mL
g
mL
mL
g
mL
3
Next, the chemist measures the volume of the unknown liquid as 1049. cm and the mass of the unknown liquid as 1.07 kg.
Calculate the density of the liquid. Round
your answer to 3 significant digits.
Given the data above, is it possible to
identify the liquid?
If it is possible to identify the liquid, do so.
O O O
g/mL
yes
no
ethanolamine
pentane
chloroform
acetone
glycerol
x10
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning