A chemistry graduate student is given 250. mL of a 0.10M propanoic acid (HC₂H,CO₂) solution. Propanoic acid is a weak acid with K=1.3 x 105. What mass of NaC₂H₂CO₂ should the student dissolve in the HC₂H5CO₂ solution to turn it into a buffer with pH = 5.31? You may assume that the volume of the solution doesn't change when the NaC₂H,CO₂ is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits. 0
A chemistry graduate student is given 250. mL of a 0.10M propanoic acid (HC₂H,CO₂) solution. Propanoic acid is a weak acid with K=1.3 x 105. What mass of NaC₂H₂CO₂ should the student dissolve in the HC₂H5CO₂ solution to turn it into a buffer with pH = 5.31? You may assume that the volume of the solution doesn't change when the NaC₂H,CO₂ is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits. 0
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter17: Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria
Section: Chapter Questions
Problem 89GQ: A buffer solution is prepared by dissolving 1.50 g each of benzoic acid, C6H5CO2H, and sodium...
Related questions
Question
Help with the following question ( use kg for the mass unit) and round the answer to 2 sig figs
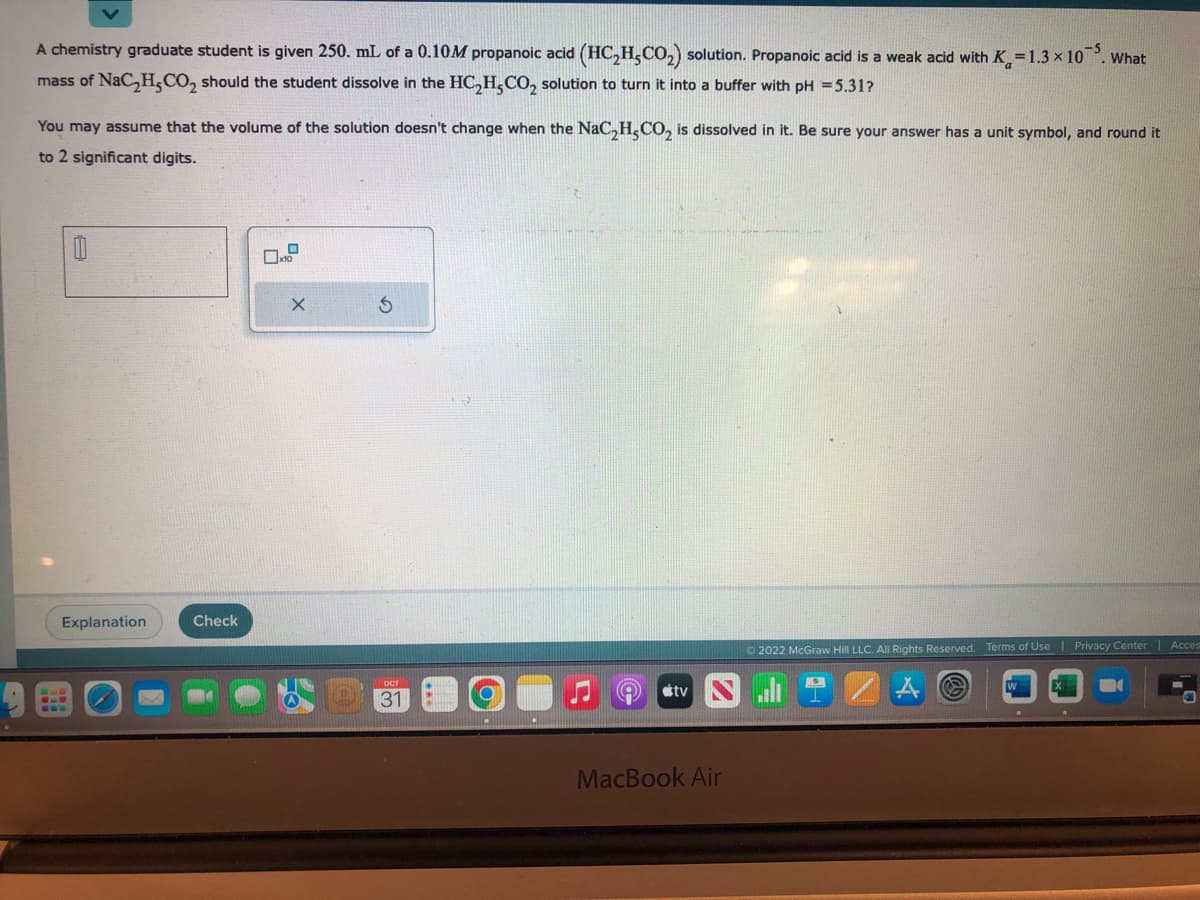
Transcribed Image Text:A chemistry graduate student is given 250. mL of a 0.10M propanoic acid (HC₂H₂CO₂) solution. Propanoic acid is a weak acid with K=1.3 × 10. What
mass of NaC₂H,CO₂ should the student dissolve in the HC₂H5CO₂ solution to turn it into a buffer with pH = 5.31?
You may assume that the volume of the solution doesn't change when the NaC₂H₂CO₂ is dissolved in it. Be sure your answer has a unit symbol, and round it
to 2 significant digits.
Explanation
Check
31
O
tv
MacBook Air
Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Acces
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning
