A copper ball of mass 100 g is at a temperature T. It is dropped in a copper calorimeter of mass 100 g, filled with 170 g of water at room temperature. Subsequently, the temperature of the system is found to be 75°C. T is 30°C, specific heat of copper (Given, room temperature = = 0.1 cal/g °C) (a) 885°C (d) 800°C (b) 1250°C (c) 825°C
A copper ball of mass 100 g is at a temperature T. It is dropped in a copper calorimeter of mass 100 g, filled with 170 g of water at room temperature. Subsequently, the temperature of the system is found to be 75°C. T is 30°C, specific heat of copper (Given, room temperature = = 0.1 cal/g °C) (a) 885°C (d) 800°C (b) 1250°C (c) 825°C
Related questions
Question
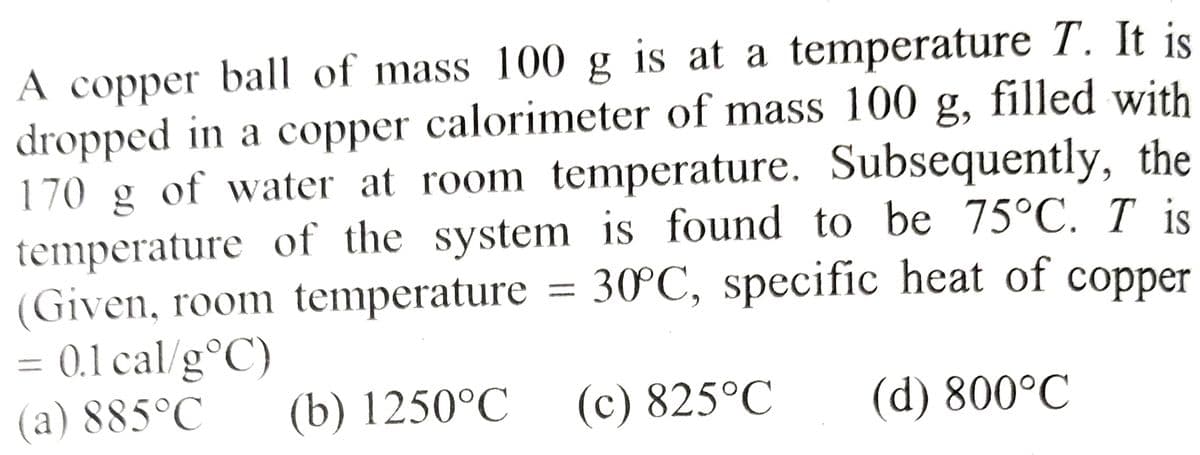
Transcribed Image Text:A copper ball of mass 100 g is at a temperature T. It is
dropped in a copper calorimeter of mass 100 g, filled with
170 g of water at room temperature. Subsequently, the
temperature of the system is found to be 75°C. I is
(Given, room temperature = 30°C, specific heat of copper
= 0.1 cal/g °C)
(a) 885°C
(b) 1250°C
(d) 800°C
(c) 825°C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps
