A current of 11.8 A is applied to 1.25 L of a solution of 0.549 mol L- aqueous HBr converting some of the H+ (aq) to H2 (g), which bubbles out of solution.
A current of 11.8 A is applied to 1.25 L of a solution of 0.549 mol L- aqueous HBr converting some of the H+ (aq) to H2 (g), which bubbles out of solution.
Chapter21: Potentiometry
Section: Chapter Questions
Problem 21.3QAP
Related questions
Question
100%
Can someone please help with question with question 8
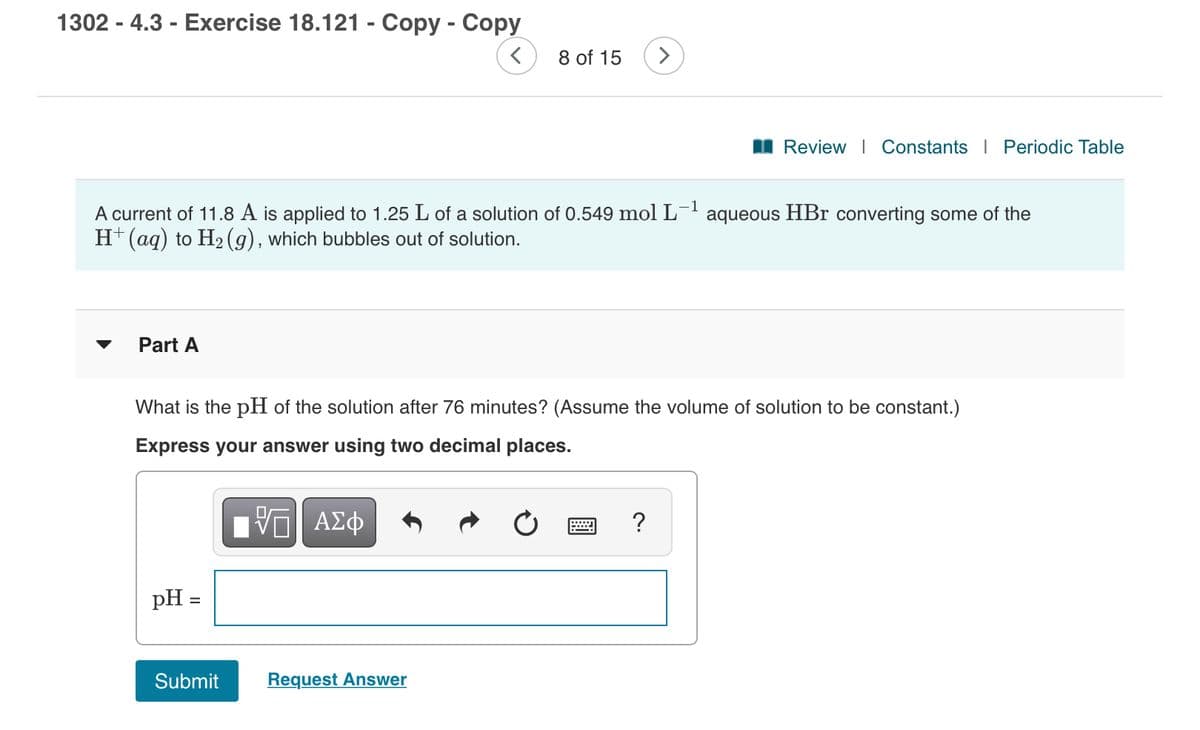
Transcribed Image Text:1302 - 4.3 - Exercise 18.121 - Copy - Copy
8 of 15
I Review | Constants I Periodic Table
A current of 11.8 A is applied to 1.25 L of a solution of 0.549 mol L¯' aqueous HBr converting some of the
H+(aq) to H2 (g), which bubbles out of solution.
Part A
What is the pH of the solution after 76 minutes? (Assume the volume of solution to be constant.)
Express your answer using two decimal places.
ΑΣφ
pH =
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning