A cyclic process is a process that return a system to its original state. Consider the following cyclic process of a mole of ideal gas with initial temperature, pressure and volume of To, Po and Vo respectively. A B: Isothermal compression to decrease its volume to Vo BC: Isobaric process to change its temperature to 2To C D: Isothermal process to decrease its pressure back to Po. D A: Isobaric process back to its original state. a Draw the PV diagram for the cyclic points. (Note: The points A,B,C,D must have the correct values of pressure and volume.) Calculate the work done, heat exchange and change in internal energy for each process. Write the final answer in terms of R and To (or Po and Vo). Calculate the total work done and heat transfer in the whole cycle.
A cyclic process is a process that return a system to its original state. Consider the following cyclic process of a mole of ideal gas with initial temperature, pressure and volume of To, Po and Vo respectively. A B: Isothermal compression to decrease its volume to Vo BC: Isobaric process to change its temperature to 2To C D: Isothermal process to decrease its pressure back to Po. D A: Isobaric process back to its original state. a Draw the PV diagram for the cyclic points. (Note: The points A,B,C,D must have the correct values of pressure and volume.) Calculate the work done, heat exchange and change in internal energy for each process. Write the final answer in terms of R and To (or Po and Vo). Calculate the total work done and heat transfer in the whole cycle.
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
Answer the problem below with its subparts a,b and c
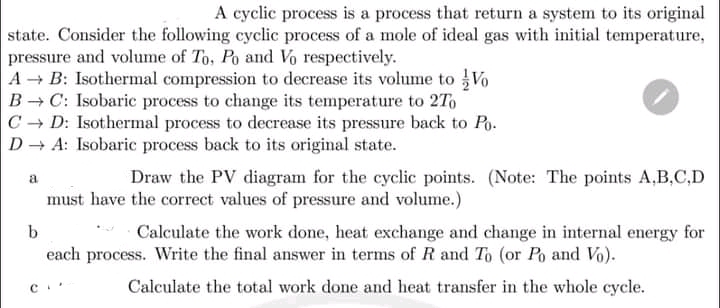
Transcribed Image Text:A cyclic process is a process that return a system to its original
state. Consider the following cyclic process of a mole of ideal gas with initial temperature,
pressure and volume of To, Po and Vo respectively.
A B: Isothermal compression to decrease its volume to Vo
B C: Isobaric process to change its temperature to 2To
C - D: Isothermal process to decrease its pressure back to Po.
D + A: Isobaric process back to its original state.
Draw the PV diagram for the cyclic points. (Note: The points A,B,C,D
a
must have the correct values of pressure and volume.)
Calculate the work done, heat exchange and change in internal energy for
each process. Write the final answer in terms of R and To (or Po and Vo).
Calculate the total work done and heat transfer in the whole cycle.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 7 steps with 7 images

Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY