Q: he alkenes shown below, which would product the product shown below, upon treatment with ozone (03),…
A: Alkenes react with O3,Zn , Acetic acid and produced aldehyde and ketone
Q: Draw all diastereomers and enantiomers for this compound.
A: Well the given compound has two chiral centers. So total number of stereoisomers is 2n=22=4 So we…
Q: What are possible units for the rate of a reaction? Multiple Choice Tu kes the World-CMS»EJ HEATHER
A:
Q: 1) BH₂ 2) H₂O₂, NaOH H₂O Cl₂ H₂O Ol OH OH
A: These reactions are addition reactions where different types of addition product forms.
Q: There should be 2 products
A: Ring opening of epoxide to give 1,2-diol. The chemical reaction is as follows:
Q: For each of the following questions, please provide a route that could reasonably be expected…
A: Given The conversion will occur in two step
Q: Derive the thermodynamic equation of state (SP)₁ = V (BP - aT)
A: Here we are required to find the thermodynamics equation of state.
Q: SO3 ő Formula PH3 8 CO SO4²- SCN¹- Number of Valence Electrons Lewis Structure
A: we have to complete the given table for the Lewis structures of the given molecules and ions
Q: The air at sea level is "80% oxygen and "20% nitrogen. True or False True False
A: Air is a mixture of various kinds of gases such as nitrogen, oxygen, inert gas,CO2 etc at different…
Q: A CD player and its battery together do 500 kJ of work, and the battery also releases 250 kJ of…
A:
Q: A chemist prepares a solution of nickel(II) chloride (NiC1₂) by measuring out 122. g of nickel(II)…
A:
Q: 4,7 Describe the composition and properties of the different types of steel.
A: There are different types of steel which are listed below --> Stainless steel Carbon steel…
Q: A flashbulb of volume 2.00 mL contains O2(g) at a pressure of 2.30 atm and a temperature of 20.0 °C.…
A: Mole is equal to weight of substance divide by it's molar mass
Q: Suppose 125 g of NO3- flows into a swamp each day. What volume of N2 would be produced each day at…
A:
Q: Predict the product of this reaction. A B OC D A) Br CN D) 1. KCN 2. H30, heat B) OH CO₂H n ? NH₂…
A: Organic reactions are those in which organic reactant react to form organic products. In the given…
Q: What is the major product from the following reaction? I Cl Y II + C1 I-Cl III Cl ?? Cl IV
A:
Q: 16.41 Explain how an acid-base indicator works in a titra- tion. What are the criteria for choosing…
A: We need to explain the working principle of acid-base indicators and the criteria for choosing an…
Q: trans-3-hexene and cis-3-hexene differ in one of the following ways. Which one? A) Products of…
A:
Q: ) Calculate absorbance of above solution? iii) If the concentration is 0.0802 M, what is the…
A:
Q: Which of the following alkyl halides will react most rapidly in an E2 reaction? CH3 H₂C I C1 CH3 II…
A: E2 reaction ----> 1) tertiary alkyl halide give more rapid E2 than after secondary alkyl halide…
Q: 4.5 Predict the structure that TICI would adopt if it is known that the cation radius for Tl+ is 159…
A: We have to calculate radius ratio to predict the structure. Radius ratio=r+/r- We have to below…
Q: O ACIDS AND BASES Making qualitative estimates of pH change or componen(check all that apply) acidic…
A:
Q: a) Can these two molecules shown below be distinguished by mass spectrometry? Give reasons for your…
A: According to Q&A guidelines of Bartleby, we are supposed to answer only the first question out…
Q: The equilibrium constant for the “water gas” reaction: C(s)+H2O(g) CO(g)+H2(g) k=2.6 at…
A:
Q: Br
A:
Q: 4. Find the molarity of a MgSO4 (FW = 120.37 g/mol) in a 2.354g/100 mL
A:
Q: What temperature (in °C) did an ideal gas shift to if it was initially at -16.0 °C at 4.62 atm and…
A:
Q: Elements in the same row of the periodic table exhibit similar chemical properties. True False
A: In the periodic table, the elements are arranged according to their atomic number (electronic…
Q: Propose an efficient synthesis for the following transformation: OH OH The transformation above can…
A:
Q: dentify the substance oxidized, substance reduced, oxidizing agent and reducing agent: MnO2 + 4 HCI→…
A: Well, we know the species in the redox reaction which accepts electrons to reduce its oxidation…
Q: Determine the pH of a 0.375M solution of C5H5NHBr (pyridinium bromide). Kb table is needed to…
A:
Q: Each value represents a different aqueous solution at 25 °C. Classify each solution as acidic,…
A: 0-7 = acidic 7-14= basic 7 = neutral
Q: One mole of gas expands isothermically to double its volume. If the gas temperature is 320 K, what…
A: Temperature (T) = 320 K Number of moles = 1mol Volume(V2) = 2 volume (V1)
Q: Topic: Reaction Kinetics Atropine base decomposes with a rate constant of 0.016/s at 40°C. The…
A:
Q: Complete the following oxidation reaction by drawing a structural formula for the product.…
A:
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A: Ionic compound are the compounds which are formed when the metal and non-metals reacts with each…
Q: You have 2.00 L of a solution containing Au*. In order to recover most of the gold(I) as an…
A:
Q: Which of the following statements is/are true? I) For a given process, AH must be zero when external…
A: The relationship among △H, ∆E and work( p∆V) is △H = △E+p△V When external pressure is zero, work is…
Q: Which of the following carbocations is(are) likely to undergo rearrangement? (A) I (B) II H₂C I CH3…
A:
Q: what is the molality
A:
Q: b) Balance the following chemical reaction, which was carried out in acidic medium. The redox…
A:
Q: Explain the importance of solubility in drug product formulation?
A: Solution is made up of two components: solute and solvent. The component which is present in major…
Q: If 0.42 mg calcium fluoride, CaF2 is used as above to add fuoride to the drinking water, does CaFz…
A: Given that , Volume of water = 250 mL Weight of CaF2 and NaF are same i.e.0.42mg = 0.00042 g…
Q: What is the role of each reagent in the preparation of a-chloro-2,6-dimethylacetanilide?…
A: This is carbonyl addition reaction where -NH2 attacks on carbonyl of acid chloride to give acid…
Q: CaO(s) + 2HCI(g) → CaCl₂(s) + H₂O(l) AS⁰ = C₂H4(g) + H₂(g) →C₂H6(g) AS⁰ = J/K J/K
A: Using the provided standard entropy values of some substance, we can determine the entropy of…
Q: 3. Determine which nuclide will be unstable. justify your answer, and give the nuclear" reaction for…
A: Elements having greater (neutron /protons) ratio has a tendency to undergoes radioactive decay…
Q: Determine [OH-] in a solution where [Ba(OH)2] = 0.0249 M.
A: Strong Base - A base which completely dissociates into it's ions in aqueous solution called strong…
Q: What is the temperature (in K) of a sample of nitrogen with an root-mean-square velocity of 612.0…
A: we have to calculate the temperature of the sample of nitrogen in K
Q: The piperazine content of an impure commercial material can be determined by precipitating and…
A:
Q: You need to make 13 mL of a 66 mM solution of a compound with FW 128.96. How many milligrams of the…
A: Given, Note: 1 mM = 10-3 M and 1 mL = 10-3 L Concentration of the solution = 66 mM = 0.066 M = 0.066…
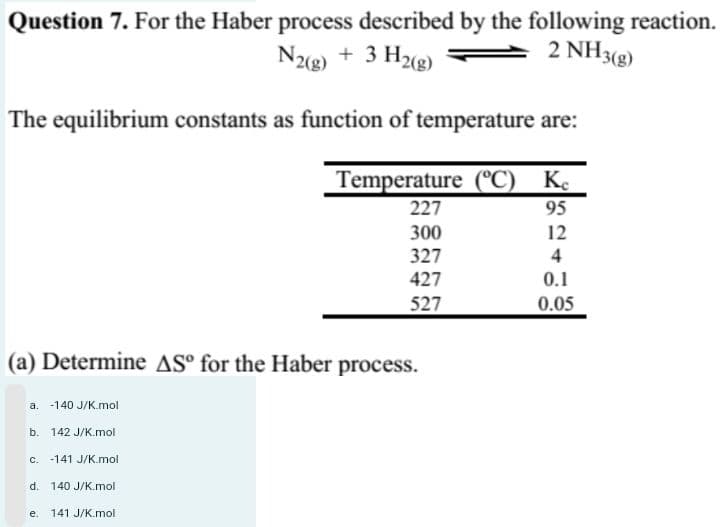
Step by step
Solved in 5 steps with 5 images

- The following questions refer to the following reaction at constant 25°C and 1 atm.2Fe(s) + (3/2)O2(g) + 3H2O(l) 2Fe(OH)3(s) H = –789 kJ/molSubstance S° (J/mol K)Fe(OH)3(s) 107Fe(s) 27O2(g) 205H2O(l) 70a. Determine Ssurr for the reaction (in kJ/mol K)b. Determine Suniv for the reaction (in kJ/mol K)2.65c. What is the G for this reaction?d. Calculate the equilibrium constant(i) In the contact process: DH = -197 kJ mol-1 Predict the position of equilibrium when: (a) temperature is decreased and (b) pressure is decreased (c) temperature is increased and (d) pressure is decreased (ii) Explain the reasoning for using compromise temperature and pressure in industrial processes such as the Contact Process5.0 g of brass (Cp = 0.38 J/g-oC, initially at 68oC) and 3.5 g of aluminum (Cp = 0.9 J/g-oC, initially at 83oC) are dropped into 20 g of water (Cp = 4.184 J/g-oC, initially at 23oC). Based on the described system, which of the following statements is/are correct? Check all that apply. The equilibrium temperature will be greater than 23oC but less than 83oC. The water will evaporate. For each material, the heat involved is sensible heat. The temperature of the aluminum will decrease. The temperature of the brass will increase.
- Which of the systems described below give homogeneous equilibria? (Select all that apply.) a. CO2(g) + H2(g) ---> CO(g) + H2O(g) b. CS2(g) + 3 O2(g) ---> CO2(g) + 2 SO2(g) c. 2 NO(g) + O2(g) ----> 2 NO2(g) d. H2O2(l) ---> H2(g) + O2(g) e. 4 NH3(g) + 5 O2(g) ---> 4 NO(g) + 6 H2O(g) f. 4 NO2(g) + O2(g) ---> 2 N2O5(g) g. 2 H2(g) + O2(g) ---> 2 H2O(l) h. 2 LiHCO3(s) ---> Li2CO3(s) + CO2(g) + H2O(g) Which give heterogeneous equilibria? (Select all that apply.) a. CO2(g) + H2(g)---> CO(g) + H2O(g) b. CS2(g) + 3 O2(g) ---> CO2(g) + 2 SO2(g) c. 2 NO(g) + O2(g) ---> 2 NO2(g) d. H2O2(l) ---> H2(g) + O2(g) e. 4 NH3(g) + 5 O2(g) ---> 4 NO(g) + 6 H2O(g) f. 4 NO2(g) + O2(g) ---> 2 N2O5(g) g. 2 H2(g) + O2(g) ---> 2 H2O(l) h. 2 LiHCO3(s) ---> Li2CO3(s) + CO2(g) + H2O(g)For the following reaction: 2 N2O5(g) 4 NO2(g) + O2(g), the value of ΔG° is −29.0 kJ/mol. What is the value of ΔG for this reaction at 350 K when [N2O5] = 0.602 M, [NO2] = 0.305 M, and [O2] = 2.10 10−2 M ? A. −2.21 × 104 kJ/mol D. −46.2 kJ/mol B. −51.1 kJ/mol E. 6.89 103 kJ/mol C. 6.89 kJ/mol100 mmol of (NH3), 300 mmol, are introduced into a 2 L container at 127 °C of (H2) and 200 mmol of (N2). A thermometer and a pressure gauge connected to the container show that the reaction between Nitrogen and Hydrogen occurs at 127 ° C and that said reaction reaches equilibrium marking a pressure of 7.1 bar. Find the equilibrium constant Kp °, considering that ?G ° 400K = −11.92 kj/mol Find the amount of substance (in mmol) that will be had for each species when the reaction has reached equilibrium.
- For the reaction COCl2g(?) ↔ ?o (?) + ?l22(?), Kp was experimentally determined to be 0.0444 at 394.8 °C. assume that initially, n moles of COCl2(g) and no moles of CO(g) and Cl2(g) are present.a) Find the partial pressures of CO (g) and Cl2 (g) at equilibrium given 1.00 bar total pressure.b) What is the value of ∆rG at equilibrium?c) Determine ∆?G using the given equilibrium constant K.Given the reaction that occurs in a closed system and is found to be at equilibrium. Typically, the reaction is favored to the right. The reaction is carried out at 250K. CH3CH2OH + 3O2 2CO2 + 3H2O ∆H = –1,154 kJ/mol ∆S = +0.35kJ/mol•K What type of chemical reaction is this? If more CH3CH2OH is added to the system, how will the reactions shift to reach equilibrium again? If water is extracted from the system, how will the reactions shift to reach equilibrium again? If heat is removed from the system, how will the reactions shift to reach equilibrium again? What is the name of the principle that helps you predict each of these shifts in equilibrium? What is the value of Gibbs free energy? Is it spontaneous?At 415 K, ∆G = -30.9 kJ/mol for the reaction 2 A (g) → B (g). If the partial pressures of A and B are 2.50 atm and 0.650 atm respectively, what is ∆G° for this reaction?
- A 348.0 g block of Cu at 639.0°C is plunged into 1.47 kg of water (T = 27.4 °C) in an insulatedcontainer. What will be the final equilibrium T of the water and the Cu? (cCu = 0.385 J g-1°C1, cH2O = 4.184 J g-1°C-1)A metal salt with the formula MCl2MCl2 crystallizes from water to form a solid with the composition MCl2⋅6H2OMCl2⋅6H2O. The equilibrium vapor pressure of water above this solid at 298 KK is 17.5 mbarmbar . hat is the value of ΔrGΔr� for the reaction MCl2⋅6H2O(s)⇌MCl2(s)+6H2O(g)MCl2⋅6H2O(�)⇌MCl2(�)+6H2O(�) when the pressure of water vapour is 17.5 mbarmbar ? Express your answer as an integer with the appropriate units.(a) In the Nernst equation, what is the numerical valueof the reaction quotient, Q, under standard conditions?(b) Can the Nernst equation be used at temperatures otherthan room temperature?

