A diesel engine does not use spark plugs to ignite the fuel and air in the cylinders. Instead, the temperature required to ignite the fuel occurs because the pistons compress the air in the cylinders. Suppose air at an initial temperature of 26.9 °C is compressed adiabatically to a temperature of 671 °C. Assume the air to be an ideal gas for which =. Find the compression ratio, which is the ratio of the initial volume to the final volume. Number Units
A diesel engine does not use spark plugs to ignite the fuel and air in the cylinders. Instead, the temperature required to ignite the fuel occurs because the pistons compress the air in the cylinders. Suppose air at an initial temperature of 26.9 °C is compressed adiabatically to a temperature of 671 °C. Assume the air to be an ideal gas for which =. Find the compression ratio, which is the ratio of the initial volume to the final volume. Number Units
Chapter3: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 98CP: In a diesel engine, the fuel is ignited without a spark plug. Instead, air in a cylinder is...
Related questions
Question
100%
The second image shows how I worked this out. I don't see how I'm getting this wrong. Thank you for your help.

Transcribed Image Text:671 +273
1
7
5
26.9 + 273
-
1
= 4.513171057
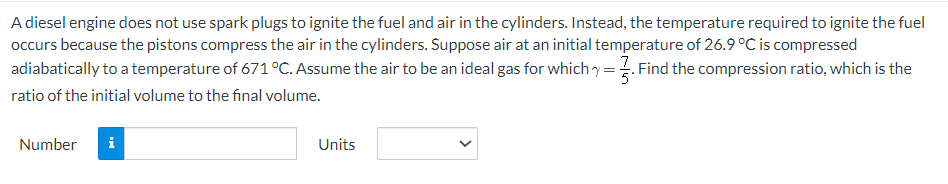
Transcribed Image Text:A diesel engine does not use spark plugs to ignite the fuel and air in the cylinders. Instead, the temperature required to ignite the fuel
occurs because the pistons compress the air in the cylinders. Suppose air at an initial temperature of 26.9 °C is compressed
adiabatically to a temperature of 671 °C. Assume the air to be an ideal gas for which y = 3. Find the compression ratio, which is the
ratio of the initial volume to the final volume.
Number
i
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning