A dramatic classroom demonstration involves cooling a balloon from room temperature (294 K) to liquid nitrogen temperature (77 K) Part A If the initial volume of the balloon is 3.2 L, what is its volume after it cools? (Ignore the effect of any gases that might iquety upon cooling) Express your answer in liters to two significant figures. ? V = L.
A dramatic classroom demonstration involves cooling a balloon from room temperature (294 K) to liquid nitrogen temperature (77 K) Part A If the initial volume of the balloon is 3.2 L, what is its volume after it cools? (Ignore the effect of any gases that might iquety upon cooling) Express your answer in liters to two significant figures. ? V = L.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section8.4: Gas Density, Molar Mass, And The Ideal Gas Law
Problem 8.9CE
Related questions
Question
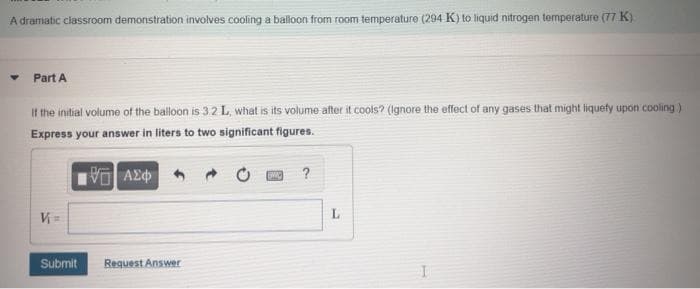
Transcribed Image Text:A dramatic classroom demonstration involves cooling a balloon from room temperature (294 K) to liquid nitrogen temperature (77 K)
• Part A
If the initial volume of the balloon is 3.2 L, what is its volume after it cools? (Ignore the effect of any gases that might liquefy upon cooling)
Express your answer in liters to two significant figures.
V =
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning