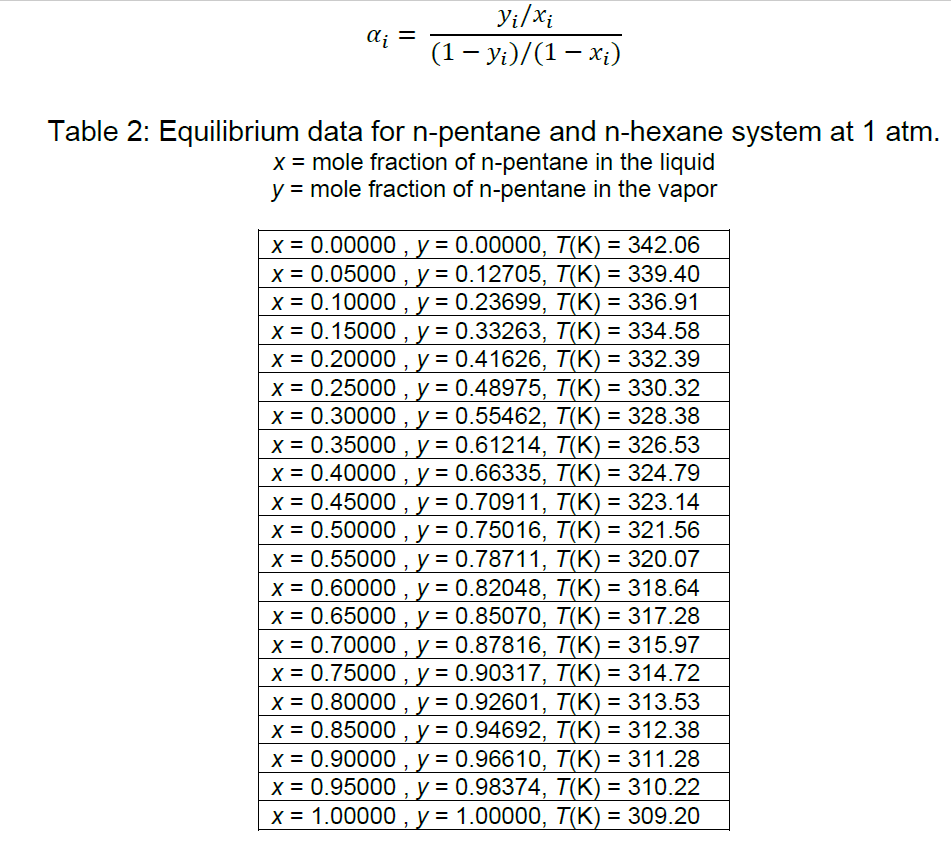
A feed of a distillation column contains 40 mol% of n-pentane and 60 mol% of n-hexane will be separated to recover 92 mol% of n-pentane as distillate at the column condition of 1 atm. The column receives a saturated liquid feed with a flow rate of 1,200 kmol/h. A total condenser is used and reflux is a saturated liquid. Bottoms from the column contains 94 mol% of n-hexane from the feed. The carbon steel column is equipped with carbon steel sieve trays which have efficiency of 43%. You need to find densities for vapour of distillate by using the chemical engineering principles that you have learnt. Use data in Table 2 and equation below to determine relative volatility of component.
A feed of a distillation column contains 40 mol% of n-pentane and 60 mol% of n-hexane will be separated to recover 92 mol% of n-pentane as distillate at the column condition of 1 atm. The column receives a saturated liquid feed with a flow rate of 1,200 kmol/h. A total condenser is used and reflux is a saturated liquid. Bottoms from the column contains 94 mol% of n-hexane from the feed. The carbon steel column is equipped with carbon steel sieve trays which have efficiency of 43%.
You need to find densities for vapour of distillate by using the chemical engineering principles that you have learnt. Use data in Table 2 and equation below to determine relative volatility of component.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps









