A FH Escape Chemistry 106! с X + O8 https://docs.google.com/forms/d/e/1FAIpQLSfdH4Cb3Jih0g_TXnrExG4Oh2E11 NuVCU4MS9N 120% Type here to search Create a solution by dissolving 14.77 g of napthalene (nonelectrolyte, MW = 128.17 g/mol) in 155.0 g of diethyl ether, (MW = 74.12 g/mol). What is the mole fraction of napththalene in the solution? You must type the 0 before the decimal. .05223 The vapor pressure of pure ether at 22 degrees Celsius (the temperature of the lab) is 414.0 Torr. What is the vapor pressure of your solution, in Torr? 392.4 The normal boiling point of diethyl ether is 34.7 degrees Celsius. What is the boiling point of your solution in degrees Celsius? The molal boiling constant for diethyl ether is 2 16 degrees Celsius/molal W og || a (28) * * DOW ↓ ED ABP 8:35 PM 5/11/2023 x Ę
A FH Escape Chemistry 106! с X + O8 https://docs.google.com/forms/d/e/1FAIpQLSfdH4Cb3Jih0g_TXnrExG4Oh2E11 NuVCU4MS9N 120% Type here to search Create a solution by dissolving 14.77 g of napthalene (nonelectrolyte, MW = 128.17 g/mol) in 155.0 g of diethyl ether, (MW = 74.12 g/mol). What is the mole fraction of napththalene in the solution? You must type the 0 before the decimal. .05223 The vapor pressure of pure ether at 22 degrees Celsius (the temperature of the lab) is 414.0 Torr. What is the vapor pressure of your solution, in Torr? 392.4 The normal boiling point of diethyl ether is 34.7 degrees Celsius. What is the boiling point of your solution in degrees Celsius? The molal boiling constant for diethyl ether is 2 16 degrees Celsius/molal W og || a (28) * * DOW ↓ ED ABP 8:35 PM 5/11/2023 x Ę
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter13: The Chemistry Of Solutes And Solutions
Section13.7: Colligative Properties Of Solutions
Problem 13.16E: Suppose that you are closing a cabin in the north woods for the winter and you do not want the water...
Related questions
Question
Transcribed Image Text:!
A
Escape Chemistry 106!
C
Type here to search
X +
https://docs.google.com/forms/d/e/1FAIpQLSfdH4Cb3Jih0g_TXnrExG4Oh2E11 NuVCU4MS9 120%
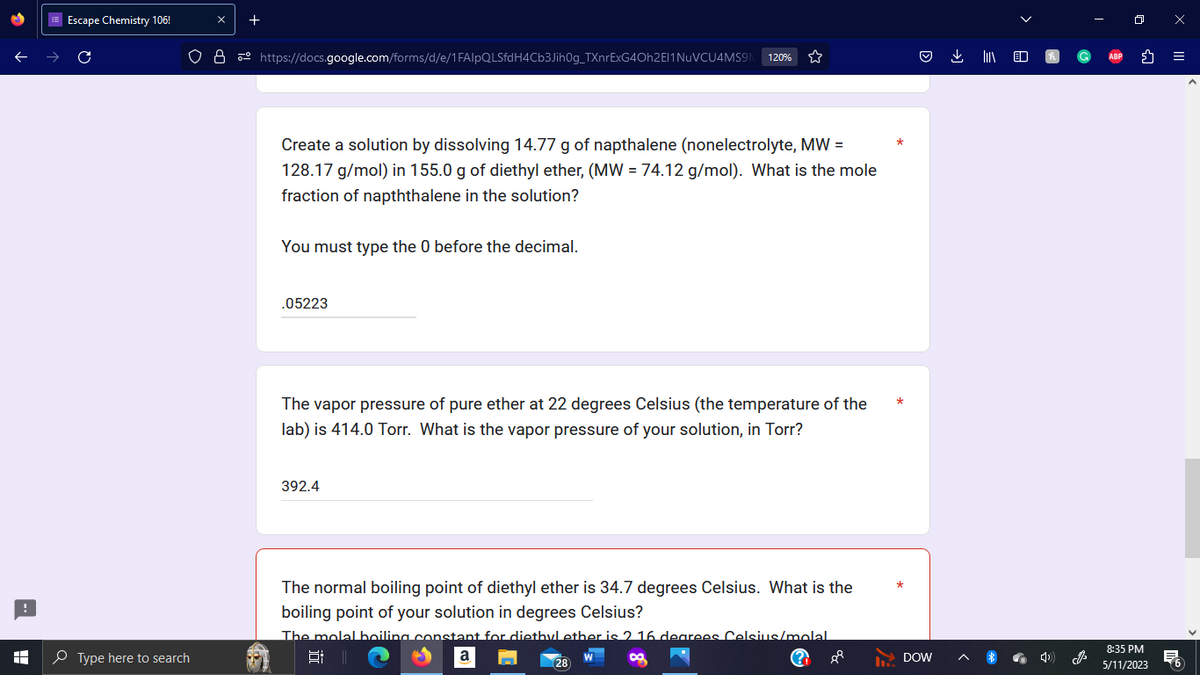
Create a solution by dissolving 14.77 g of napthalene (nonelectrolyte, MW =
128.17 g/mol) in 155.0 g of diethyl ether, (MW = 74.12 g/mol). What is the mole
fraction of napththalene in the solution?
You must type the 0 before the decimal.
.05223
The vap sure of pure ether at 22 degrees Celsius (the temperature of the
lab) is 414.0 Torr. What is the vapor pressure of your solution, in Torr?
392.4
The normal boiling point of diethyl ether is 34.7 degrees Celsius. What is the
boiling point of your solution in degrees Celsius?
The molal boiling constant for diethyl ether is 2 16 degrees Celsius/molal
a
W
||
28
op
*
Ⓒ↓||||
DOW
J
ABP
8:35 PM
5/11/2023
Transcribed Image Text:!
A
Escape Chemistry 106!
→ C
X +
O8 https://docs.google.com/forms/d/e/1FAIpQLSfdH4Cb3Jih0g_TXnrExG40h2E11 NuVCU4MS9 120%
Type here to search
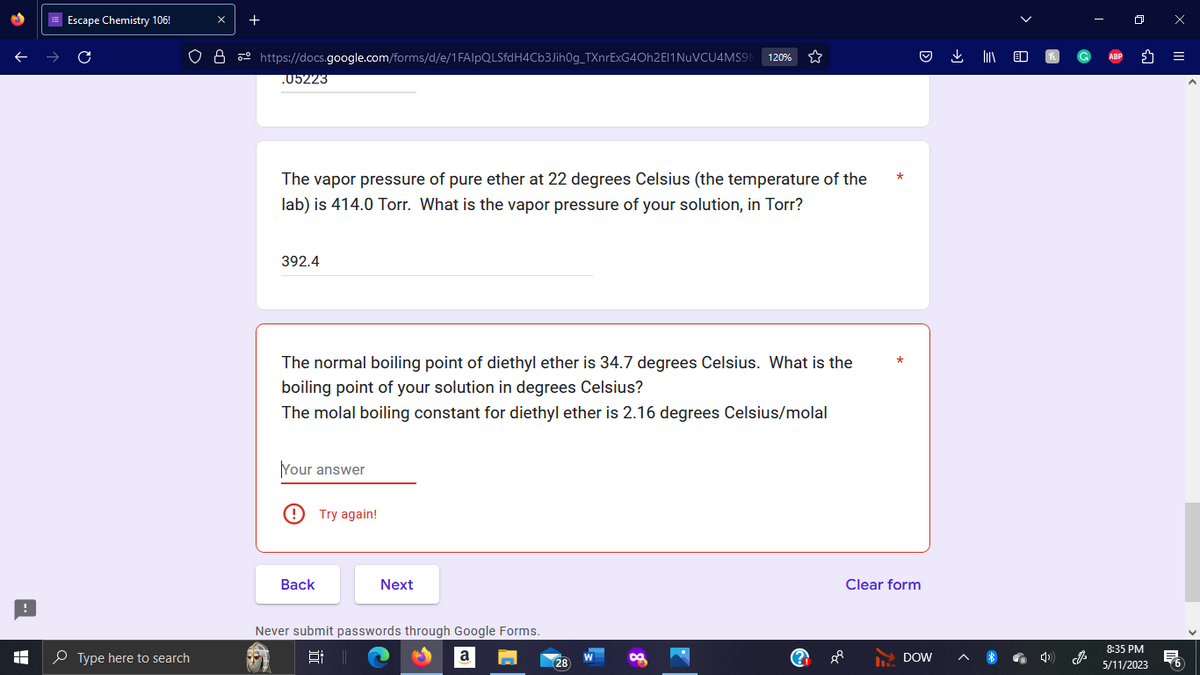
05223
The vapor pressure of pure ether at 22 degrees Celsius (the temperature of the
lab) is 414.0 Torr. What is the vapor pressure of your solution, in Torr?
392.4
The normal boiling point of diethyl ether is 34.7 degrees Celsius. What is the
boiling point of your solution in degrees Celsius?
The molal boiling constant for diethyl ether is 2.16 degrees Celsius/molal
Your answer
Back
Try again!
Next
Never submit passwords through Google Forms.
a
||
28
W
*
*
Ⓒ↓ |111\
Clear form
DOW
J
ABP
8:35 PM
5/11/2023
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning