a) For an altitude of 30 km (235 K), calculate the rate of 03 decomposition by nitric oxide for comparison with the rate of NO regeneration in the NO, catalytic cycle. b) Repeat the rate calculations at 30 km for the catalytic cycles involving HOx and CIOx. X+ O, E, / kJ mol k35 / cm molecules Concentration / A/ cm' molecules molecules cm 1.0 x 10° 2.0 x 10 1.4 x 10-10 3.9 1.9 x 101 OH 1.0 x 10 1.6 x 10-12 7.8 3.0 x 10-14 NO 5.0 x 10* 1.8 x 10-12 11.4 5.3 x 10-15 CI Very small 2.8 x 10-11 21 9.6 x 10-12
a) For an altitude of 30 km (235 K), calculate the rate of 03 decomposition by nitric oxide for comparison with the rate of NO regeneration in the NO, catalytic cycle. b) Repeat the rate calculations at 30 km for the catalytic cycles involving HOx and CIOx. X+ O, E, / kJ mol k35 / cm molecules Concentration / A/ cm' molecules molecules cm 1.0 x 10° 2.0 x 10 1.4 x 10-10 3.9 1.9 x 101 OH 1.0 x 10 1.6 x 10-12 7.8 3.0 x 10-14 NO 5.0 x 10* 1.8 x 10-12 11.4 5.3 x 10-15 CI Very small 2.8 x 10-11 21 9.6 x 10-12
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter11: Rate Of Reaction
Section: Chapter Questions
Problem 99QAP: The gas-phase reaction between hydrogen and iodine H2(g)+I22HI(g)proceeds with a rate constant for...
Related questions
Question
100%
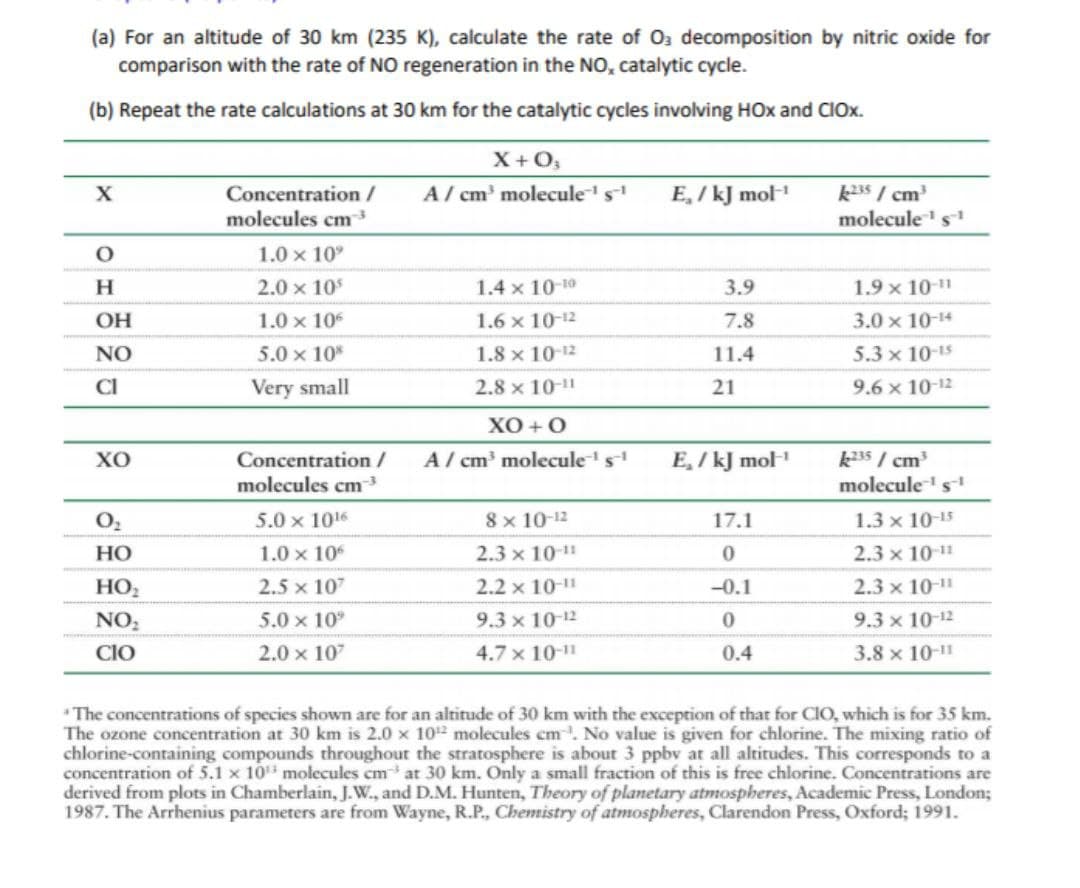
Transcribed Image Text:(a) For an altitude of 30 km (235 K), calculate the rate of Oz decomposition by nitric oxide for
comparison with the rate of NO regeneration in the NO, catalytic cycle.
(b) Repeat the rate calculations at 30 km for the catalytic cycles involving HOx and CIOx.
X+ O,
k35 / cm
molecule's
Concentration /
A/ cm' molecules
E, / kJ mol
molecules cm
1.0 x 10°
H
2.0 x 10
1.4 x 10-10
3.9
1.9 x 10-1
OH
1.0 x 10
1.6 x 10-12
7.8
3.0 x 10-14
NO
5.0 x 10*
1.8 x 10-12
11.4
5.3 x 10-15
CI
Very small
2.8 x 10-11
21
9.6 x 10-12
ХО + О
хо
Concentration /
A / cm' molecule's
E, / kJ mol
k35 / cm
molecules cm
molecules
5.0 x 1016
8 x 10-12
17.1
1.3 x 10-15
НО
1.0 x 10
2.3 x 10-11
2.3 x 10-11
HO,
2.5 x 107
2.2 x 10-1"
-0.1
2.3 x 10-
NO,
5.0 x 10°
9.3 x 10-12
9.3 x 10-12
CIO
2.0 x 107
4.7 x 10-11
0.4
3.8 x 10-1
" The concentrations of species shown are for an altitude of 30 km with the exception of that for CIO, which is for 35 km.
The ozone concentration at 30 km is 2.0 x 102 molecules cm. No value is given for chlorine. The mixing ratio of
chlorine-containing compounds throughout the stratosphere is about 3 ppbv at all altitudes. This corresponds to a
concentration of 5.1 x 10 molecules cm at 30 km. Only a small fraction of this is free chlorine. Concentrations are
derived from plots in Chamberlain, J.W., and D.M. Hunten, Theory of planetary atmospheres, Academic Press, London;
1987. The Arrhenius parameters are from Wayne, R.P., Chemistry of atmospheres, Clarendon Press, Oxford; 1991.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
