(a) For the reaction of 1-methoxy-4-methylbenzene with CH3C(O)CI in the presence of AICI3, describe the bonding and electron distribution in 1-methoxy- 4-methylbenzene, formation and structure of the electrophile in the reaction, and the most stable intermediate responsible for the end product. H₂C CI. I, AICI3 Me -OMe 1-methoxy-4-methylbenzene
(a) For the reaction of 1-methoxy-4-methylbenzene with CH3C(O)CI in the presence of AICI3, describe the bonding and electron distribution in 1-methoxy- 4-methylbenzene, formation and structure of the electrophile in the reaction, and the most stable intermediate responsible for the end product. H₂C CI. I, AICI3 Me -OMe 1-methoxy-4-methylbenzene
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter6: Reactions Of Alkenes
Section: Chapter Questions
Problem 6.38P: Reaction of -pinene with borane followed by treatment of the resulting trialkylborane with alkaline...
Related questions
Question
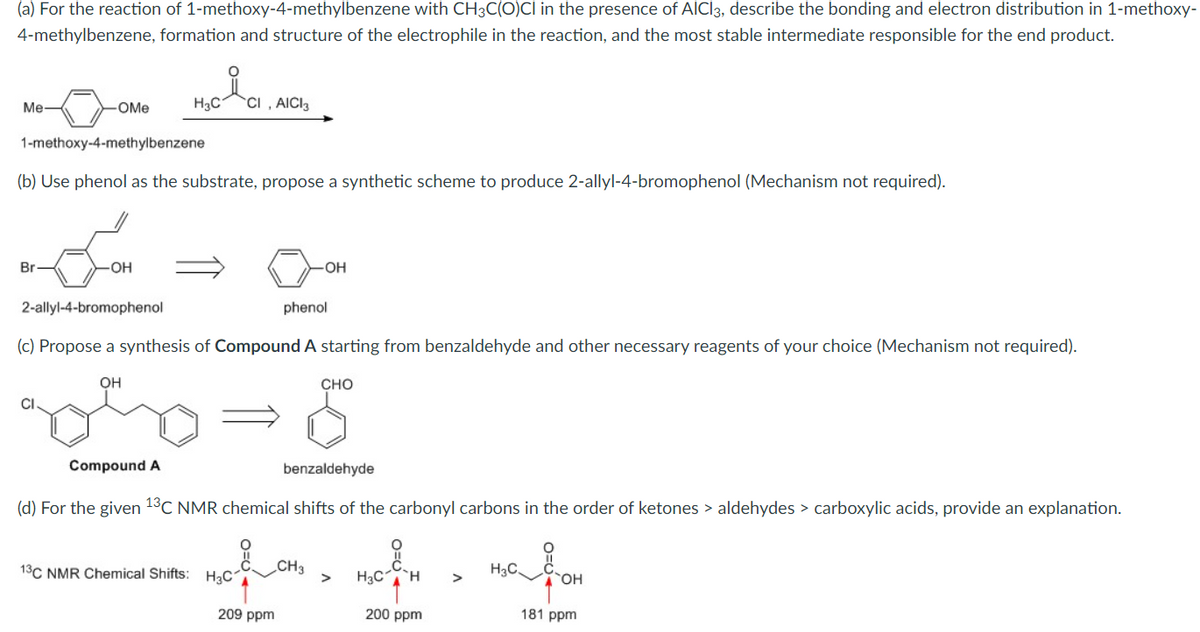
Transcribed Image Text:(a) For the reaction of 1-methoxy-4-methylbenzene with CH3C(O)CI in the presence of AICI3, describe the bonding and electron distribution in 1-methoxy-
4-methylbenzene, formation and structure of the electrophile in the reaction, and the most stable intermediate responsible for the end product.
i
H3C1 CI, AICI 3
Me-
-OMe
1-methoxy-4-methylbenzene
(b) Use phenol as the substrate, propose a synthetic scheme to produce 2-allyl-4-bromophenol (Mechanism not required).
Br
OH
OH
phenol
2-allyl-4-bromophenol
(c) Propose a synthesis of Compound A starting from benzaldehyde and other necessary reagents of your choice (Mechanism not required).
OH
CHO
CI.
Compound A
benzaldehyde
(d) For the given 13C NMR chemical shifts of the carbonyl carbons in the order of ketones > aldehydes > carboxylic acids, provide an explanation.
O
CH3
H3C.
13C NMR Chemical Shifts: H₂C1
H₂C H >
OH
200 ppm
181 ppm
209 ppm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning