Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.24QAP
Related questions
Question
100%
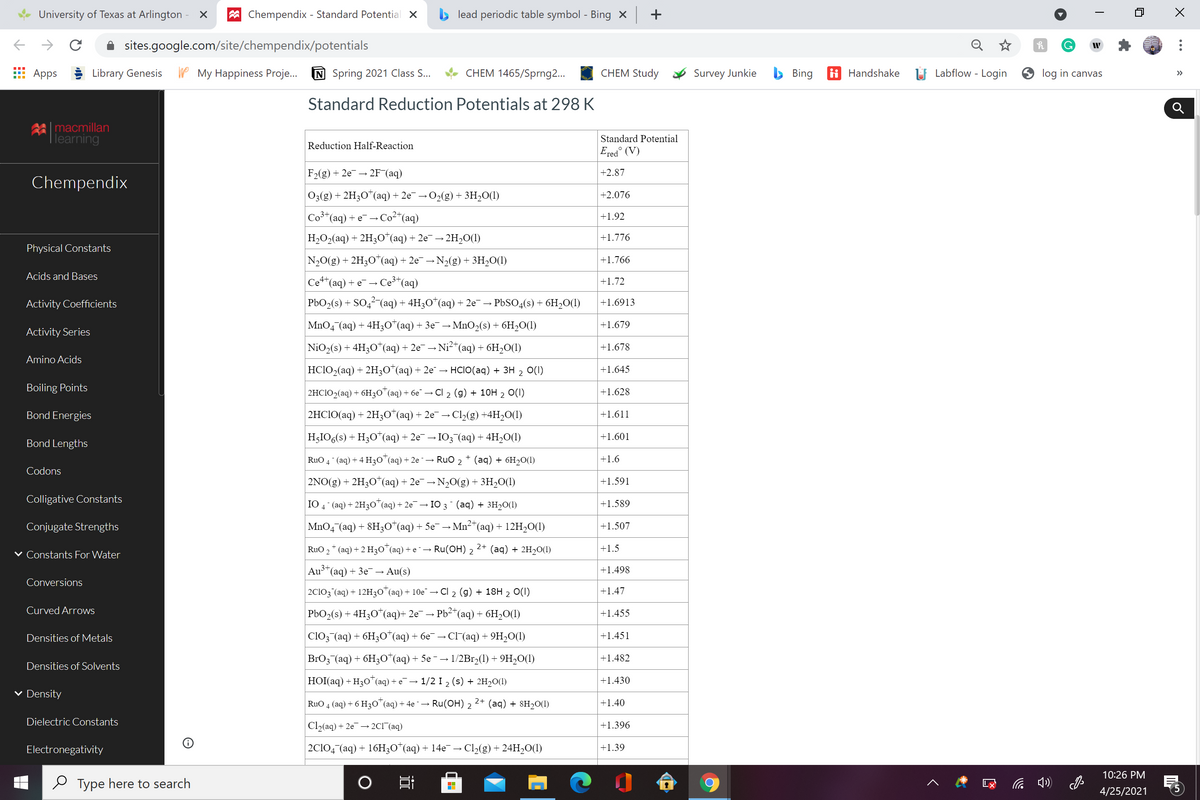
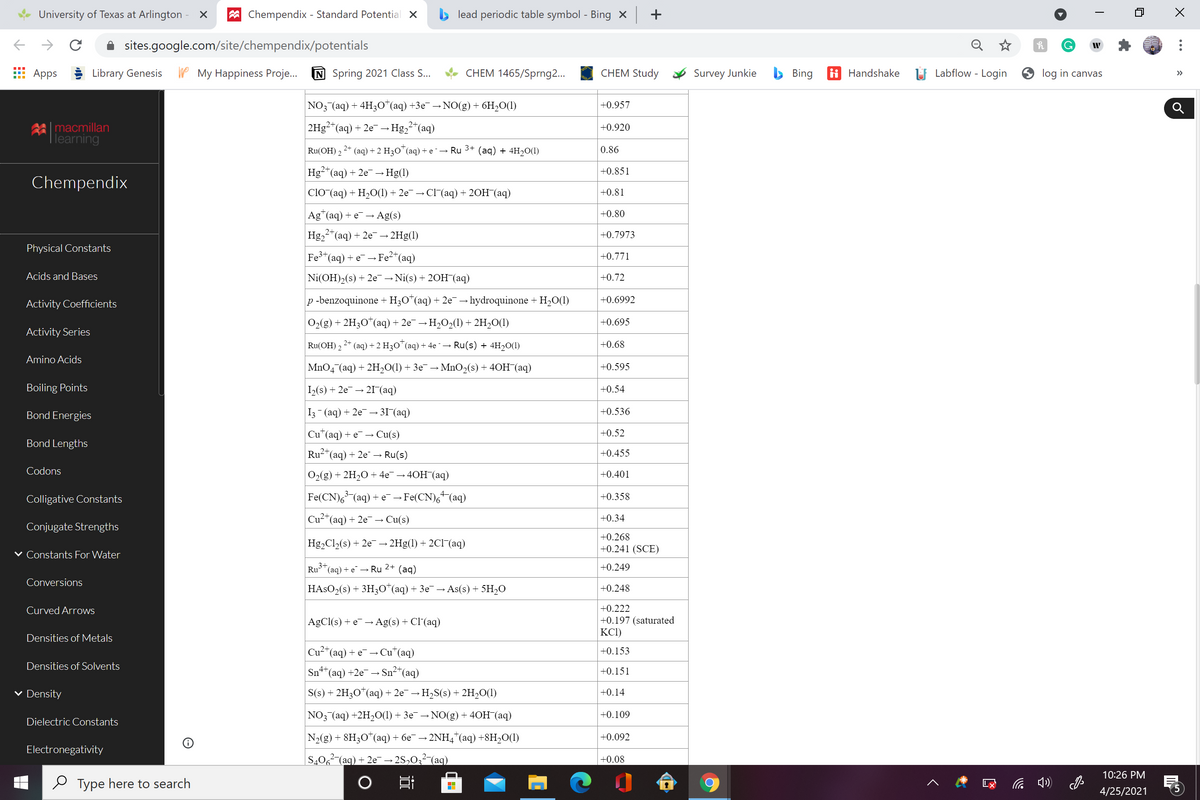
A galvanic (voltaic) cell consists of an inert platinum electrode in a solution containing 1.0 M chromium(III) ion and 1.0 M chromium(II) ion, and another inert platinum electrode in a solution containing 1.0 M cobalt(III) ion and 1.0 M cobalt(II) ion, connected by a salt bridge. Calculate the standard potential for this cell at 25°C. Standard reduction potentials can be found in the standard reduction potentials table.
Transcribed Image Text:University of Texas at Arlington - X
A Chempendix - Standard Potential X
b lead periodic table symbol - Bing x +
sites.google.com/site/chempendix/potentials
Q
Apps Library Genesis
P My Happiness Proje..
N Spring 2021 Class S...
CHEM 1465/Sprng2..
CHEM Study
Survey Junkie
b Bing
i Handshake
U Labflow - Login
6 log in canvas
>>
Standard Reduction Potentials at 298 K
a macmillan
| learning
Standard Potential
Reduction Half-Reaction
Ered° (V)
F2(g) + 2e → 2F (aq)
+2.87
Chempendix
03(g) + 2H3O"(aq) + 2e¯ → O2(g) + 3H,O(1)
|+2.076
Co3*(aq) + e → Co²*(aq)
+1.92
H,O2(aq) + 2H3O*(aq) + 2e¯ → 2H,O(1)
+1.776
Physical Constants
N20(g) + 2H3O*(aq) + 2e¯ →N2(g) + 3H2O(1)
+1.766
Acids and Bases
Cet*(aq) + e - Ce³*(aq)
+1.72
Activity Coefficients
PbO2(s) + SO,? (aq) + 4H3O*(aq) + 2e¯→ PBSO4(s) + 6H20(1)
+1.6913
MnO4 (aq) + 4H3O*(aq) + 3e¯ →MnO2(s) + 6H2O(1)
|+1.679
Activity Series
NiO2(s) + 4H;0*(aq) + 2e¯ → Ni2*(aq) + 6H,O(1)
:2+
+1.678
Amino Acids
HC1O2(aq) + 2H30*(aq) + 2e" → HCIO(aq) + 3H 2 0(I)
+1.645
Boiling Points
2HC1O2(aq) + 6H3O"(aq) + 6e¯ → CI 2 (g) + 10H 2 O(1)
+1.628
Bond Energies
2HCIO(aq) + 2H3O*(aq) + 2e¯ → Cl2(g) +4H20(1)
+1.611
H;IO6(s) + H3O*(aq) + 2e→ I03 (aq) + 4H,O(1)
+1.601
Bond Lengths
RuO 4 (aq) + 4 H30"(aq) + 2e ¯→ RuO 2 + (aq) + 6H2O(1)
+1.6
Codons
2NO(g) + 2H3O*(aq) + 2e¯ → N,0(g) + 3H2O(1)
+1.591
Colligative Constants
IO 4 (aq) + 2H30*(aq) + 2e¯ → IO 3 (aq) + 3H20(1)
+1.589
Conjugate Strengths
MnO4 (aq) + 8H30*(aq) + 5e¯ → Mn²"(aq) + 12H20(1)
+1.507
v Constants For Water
RuO ,* (aq) + 2 H3O (aq) + e ¯ → Ru(OH) 2
2+ (aq) + 2H20(1)
+1.5
Au3*(aq) + 3e¯→ Au(s)
+1.498
Conversions
2C103 (aq) + 12H30*(aq) + 10e¯ →
Cl 2 (g) + 18H 2 0(1)
+1.47
Curved Arrows
PbO2(s) + 4H3O*(aq)+ 2e¯ → Pb2*(aq) + 6H2O(1)
+1.455
Densities of Metals
C1O3 (aq) + 6H;0" (aq) + бе — СГ(aq) + 9H-0()
+1.451
BrO3 (аq) + 6H;о" (aq) + 5e - — 1/2Br>() + 9H-0(1)
+1.482
Densities of Solvents
HOI(aq) + H30*(aq) + e¯→ 1/2 I 2 (s) + 2H2O(1I)
+1.430
v Density
RuO 4 (aq) + 6 H3O*(aq) + 4e ¯→ Ru(OH) 2 2+ (aq) + 8H20(1)
+1.40
Dielectric Constants
Cl2(aq) + 2e → 2CI^(aq)
+1.396
Electronegativity
2C104 (aq) + 16H;O*(aq) + 14e¯ → Cl,(g) + 24H20(1)
+1.39
10:26 PM
O Type here to search
4/25/2021
Transcribed Image Text:University of Texas at Arlington - X
A Chempendix - Standard Potential X
b lead periodic table symbol - Bing x +
sites.google.com/site/chempendix/potentials
Q
Apps Library Genesis
P My Happiness Proje..
N Spring 2021 Class S...
CHEM 1465/Sprng2...
CHEM Study
Survey Junkie
b Bing
i Handshake
U Labflow - Login
6 log in canvas
>>
NO3 (aq) + 4H3O*(aq) +3e¯ → NO(g) + 6H2O(1)
+0.957
|2H3²*(aq)
a macmillan
| learning
+ 2e-→Hg,2"(aq)
+0.920
2+
Ru(OH) 2
(aq) + 2 H30™(aq) + e ¯→ Ru 3+
(aд) + 4H20()
0.86
Hg2"(aq) + 2e →Hg(1)
+0.851
Chempendix
CIO (aq) + H-О(1) + 2е — СI (aq) + 20H (aq)
+0.81
Ag*(aq) +
· Ag(s)
+0.80
Hg,2"(aq) + 2e - 2Hg(1)
+0.7973
Physical Constants
Fe3*(aq) + e¯ → Fe²*(aq)
+0.771
Acids and Bases
Ni(OH)2(s) + 2e¯→ Ni(s) + 20H¯(aq)
+0.72
Activity Coefficients
p -benzoquinone +H;O*(aq) + 2e¯ → hydroquinone + H20(1)
+0.6992
02(g) + 2H3O*(aq) + 2e¯ → H2O2(1) + 2H2O(1)
+0.695
Activity Series
Ru(OH) 2
2* (aq) + 2 H3O*(aq) + 4e - → Ru(s) + 4H20(1)
+0.68
Amino Acids
MnO4 (aq) + 2H,0(1) + 3e¯ → MnO2(s) + 40H (aq)
+0.595
Boiling Points
1(s) + 2e¯
→ 21 (aq)
+0.54
Bond Energies
I3- (aq) + 2e¯ – 3I^(aq)
+0.536
Cu*(aq)
+ e → Cu(s)
+0.52
Bond Lengths
Ru2*(aq) + 2e¯ → Ru(s)
+0.455
Codons
О2(g) + 2H,0 + 4е — 40H (aq)
+0.401
Colligative Constants
Fe(CN),(aq) + e → Fe(CN),+(aq)
+0.358
Cu²*(aq) + 2e¯ – Cu(s)
+0.34
Conjugate Strengths
+0.268
Hg2Cl(s) + 2e¯ →
- 2Hg(1) + 2C1(aq)
+0.241 (SCE)
v Constants For Water
3+
Ru"(aq) + e¯ – Ru 2+
(aq)
+0.249
Conversions
HASO2(s) + 3H30*(aq) + 3e¯ → As(s) + 5H2O
+0.248
Curved Arrows
+0.222
+0.197 (saturated
KCI)
AgCl(s) + e-→ Ag(s) + Cl'(aq)
Densities of Metals
Cu²*(aq) + e¯ → Cu*(aq)
+0.153
Densities of Solvents
Sn+*(aq) +2e → Sn²*(aq)
+0.151
v Density
S(s) + 2H3O"(aq) + 2e¯-
-H,S(s) + 2H,O(1)
+0.14
NO3 (aq) +2H-0() + Зе — NO(g) + 40H (аq)
+0.109
Dielectric Constants
N2(g) + 8H;O*(aq) + 6e¯ → 2NH4*(aq) +8H,O(1)
+0.092
Electronegativity
S,06²-(aq) + 2e → 2S,0;² (aq)
+0.08
10:26 PM
O Type here to search
4/25/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning