A hot mixture of acetylene (C2H2) and oxygen are allowed to combust in a sealed piston chamber. After reaction, the total number of moles of compounds is 12/13 that of the initial total number of moles of compounds. What are two possible integer values for n? 2 C,H, (g) + 5 0,(g) → 4 CO2 (g) + 2 H,0 (g) C100 C66.7 E n moles m moles 4 moles
A hot mixture of acetylene (C2H2) and oxygen are allowed to combust in a sealed piston chamber. After reaction, the total number of moles of compounds is 12/13 that of the initial total number of moles of compounds. What are two possible integer values for n? 2 C,H, (g) + 5 0,(g) → 4 CO2 (g) + 2 H,0 (g) C100 C66.7 E n moles m moles 4 moles
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.72PAE: 4.72 The picture shown depicts the species present at the start of a combustion reaction between...
Related questions
Question
!
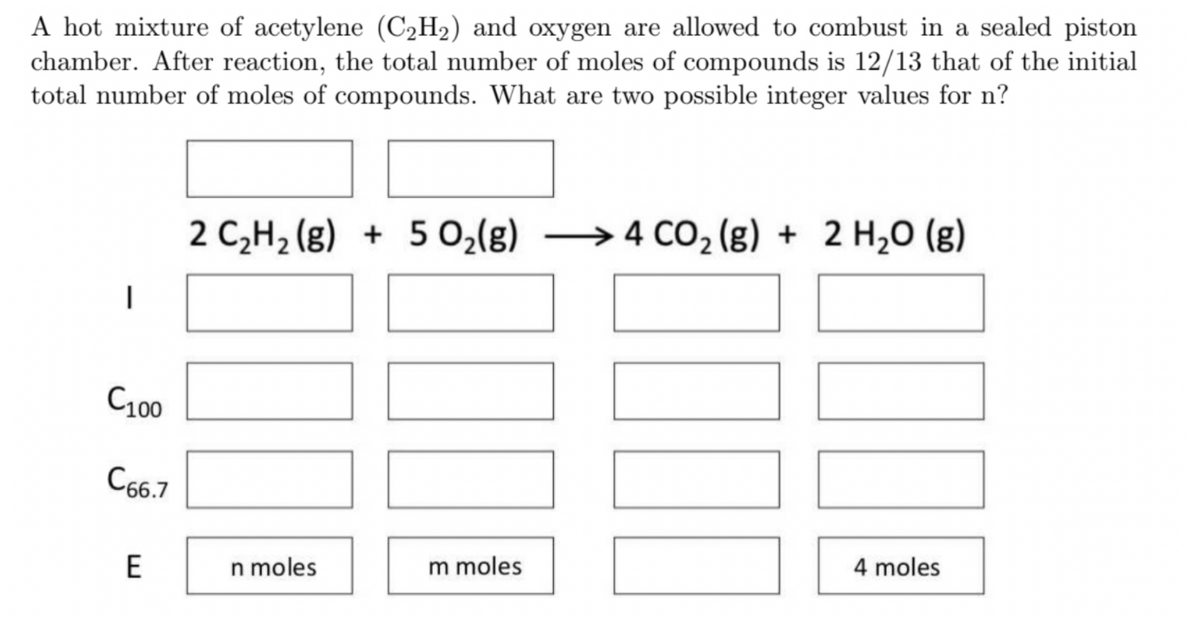
Transcribed Image Text:A hot mixture of acetylene (C2H2) and oxygen are allowed to combust in a sealed piston
chamber. After reaction, the total number of moles of compounds is 12/13 that of the initial
total number of moles of compounds. What are two possible integer values for n?
2 C,H2 (g) + 5 0,(g) →4 CO, (g) + 2 H,0 (g)
→4 CO2 (g) + 2 H,0 (g)
C100
C66.7
E
n moles
m moles
4 moles
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
