(a) (i) Calculate the ionisation energy of Be³* in eV considering that the energy levels of a hydrogenic atom are given by the following equation: En = -Z²hcRoon² where all the symbols have their usual meaning. 1 (ii) Would you expect the ionisation energy of Be³+ to be larger or smaller than the ionisation energy of the H atom? Briefly explain your answer.
(a) (i) Calculate the ionisation energy of Be³* in eV considering that the energy levels of a hydrogenic atom are given by the following equation: En = -Z²hcRoon² where all the symbols have their usual meaning. 1 (ii) Would you expect the ionisation energy of Be³+ to be larger or smaller than the ionisation energy of the H atom? Briefly explain your answer.
Related questions
Question
6
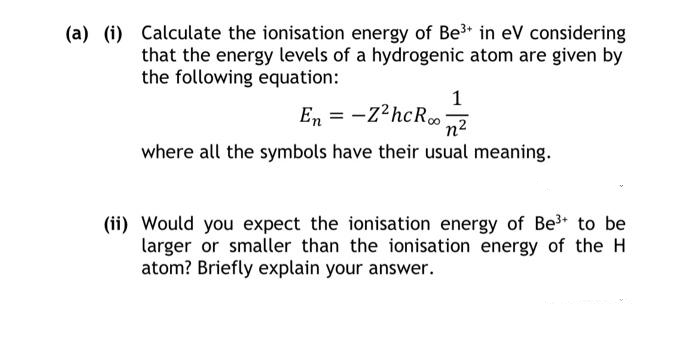
Transcribed Image Text:(a) (i) Calculate the ionisation energy of Be³+ in eV considering
that the energy levels of a hydrogenic atom are given by
the following equation:
1
En = -Z²hcR∞on²
where all the symbols have their usual meaning.
(ii) Would you expect the ionisation energy of Be³+ to be
larger or smaller than the ionisation energy of the H
atom? Briefly explain your answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps
