A) In the temperature range between 25°C and 340°C, the reaction in the previous two questions is (SEE THE PICTURE ATTACHED): Select one: a. non-spontaneous b. You can't tell about the range between 25°C and 340°C c. spontaneous B) When you increase the temperature on the reaction in the previous two questions, the amount of work needed to get the reaction to proceed will: Select one: a. increase b. be unchanged c. decrease
A) In the temperature range between 25°C and 340°C, the reaction in the previous two questions is (SEE THE PICTURE ATTACHED): Select one: a. non-spontaneous b. You can't tell about the range between 25°C and 340°C c. spontaneous B) When you increase the temperature on the reaction in the previous two questions, the amount of work needed to get the reaction to proceed will: Select one: a. increase b. be unchanged c. decrease
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 73QAP: Consider the reaction below at 25°C: 2MnO4(aq)+16H+(aq)+10Br(aq)2Mn2+(aq)+5Br2(l)+8H2O Use Table...
Related questions
Question
A)
In the temperature range between 25°C and 340°C, the reaction in the previous two questions is (SEE THE PICTURE ATTACHED):
Select one:
a. non-spontaneous
b. You can't tell about the range between 25°C and 340°C
c. spontaneous
B)
When you increase the temperature on the reaction in the previous two questions, the amount of work needed to get the reaction to proceed will:
Select one:
a. increase
b. be unchanged
c. decrease
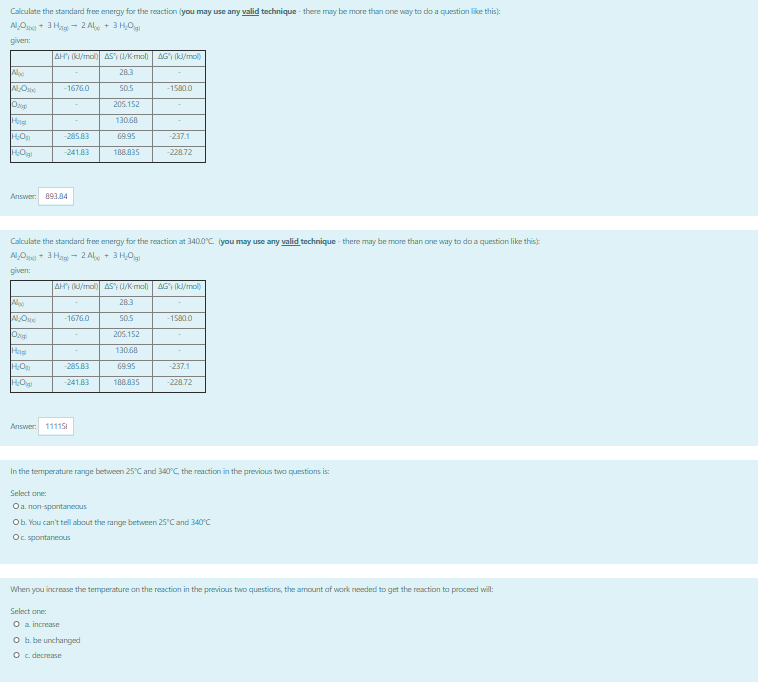
Transcribed Image Text:Calculate the standard free energy for the reaction (you may use any valid technique - there may be more than one way to do a question like this):
AOg + 3 Hag - 2 Al + 3 H,Og
given:
AH; (k/mol) AS'; U/K-mol)
AG (kl/mol)
Alo
283
-1676.0
50.5
-15800
205.152
130,68
-285.83
69.95
-237.1
-241.83
108A35
-228.72
Answer: 093.84
Calculate the standard free energy for the reaction at 340.0°C (you may use any valid technique - there may be more than one way to do a questian like this)
AlOg* 3 Hag - 2 Alo + 3 H,Og
given:
AH; (k/mol) AS'; U/K-mol)
AG (kl/mol)
28.3
-1676.0
50.5
-1580.0
205.152
130,68
-285.83
69.95
-237.1
-241.83
188А35
-228.72
Answer: 11115I
In the temperature range between 25°C and 340°C, the reaction in the previous two questions is:
Select one
Oa. non-spontaneous
Ob You can't tell about the range between 25'Cand 340°C
Oc sportaneous
When you increase the temperature on the reaction in the previous two questions, the amount af work needed to get the reaction to proceed will:
Select one:
O a increase
O b. be unchanged
O c. decrease
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning