A lab technician wants to make 500 mL of a 2.50 mol/L solution from a standard solution of concentrated sulfuric acid. Drag and drop to put the steps, of the preparation of a solution by dilution, in the correct order. Step 1 Step 2 Step 3 Step 4 Step 5 Calculate the volume needed of the concentrated sulfuric acid solution. Place 250 mL of distilled water into a 500 mL volumetric flask. Pipette the calculated volume of the concentrated sulfuric acid solution into the volumetric flask. Top up the solution with distilled water until it reaches the 500 mL meniscus line. Stopper and invert the flask to mix. Place 250 mL of distilled water into a 500 mL Erlenmeyer flask. Place 250 mL of tap water into a 500 mL Erlenmeyer flask. Place 250 mL of tap water into a 500 mL volumetric flask. Add 250 mL of distilled water to the concentrated sulfuric acid solution in the 500 mL volumetric flask. Use a graduated cylinder to measure the calculated volume of the concentrated sulfuric acid solution.
A lab technician wants to make 500 mL of a 2.50 mol/L solution from a standard solution of concentrated sulfuric acid. Drag and drop to put the steps, of the preparation of a solution by dilution, in the correct order. Step 1 Step 2 Step 3 Step 4 Step 5 Calculate the volume needed of the concentrated sulfuric acid solution. Place 250 mL of distilled water into a 500 mL volumetric flask. Pipette the calculated volume of the concentrated sulfuric acid solution into the volumetric flask. Top up the solution with distilled water until it reaches the 500 mL meniscus line. Stopper and invert the flask to mix. Place 250 mL of distilled water into a 500 mL Erlenmeyer flask. Place 250 mL of tap water into a 500 mL Erlenmeyer flask. Place 250 mL of tap water into a 500 mL volumetric flask. Add 250 mL of distilled water to the concentrated sulfuric acid solution in the 500 mL volumetric flask. Use a graduated cylinder to measure the calculated volume of the concentrated sulfuric acid solution.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter13: The Chemistry Of Solutes And Solutions
Section: Chapter Questions
Problem 28QRT
Related questions
Question
Can you give me the solution for this one please?
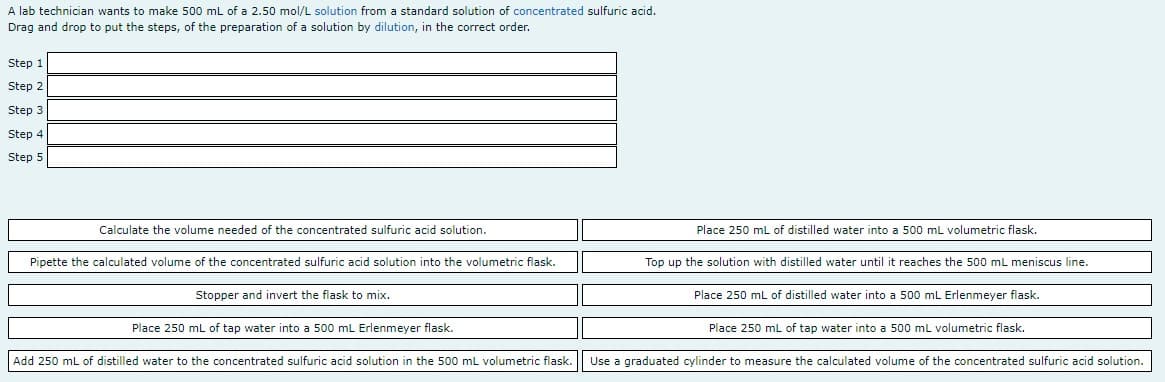
Transcribed Image Text:A lab technician wants to make 500 mL of a 2.50 mol/L solution from a standard solution of concentrated sulfuric acid.
Drag and drop to put the steps, of the preparation of a solution by dilution, in the correct order.
Step 1
Step 2
Step 3
Step 4
Step 5
Calculate the volume needed of the concentrated sulfuric acid solution.
Place 250 mL of distilled water into a 500 mL volumetric flask.
Pipette the calculated volume of the concentrated sulfuric acid solution into the volumetric flask.
Top up the solution with distilled water until it reaches the 500 mL meniscus line.
Stopper and invert the flask to mix.
Place 250 mL of distilled water into a 500 mL Erlenmeyer flask.
Place 250 mL of tap water into a 500 mL Erlenmeyer flask.
Place 250 ml of tap water into a 500 ml volumetric flask.
Add 250 mL of distilled water to the concentrated sulfuric acid solution in the 500 mL volumetric flask.
Use a graduated cylinder to measure the calculated volume of the concentrated sulfuric acid solution.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning