A laboratory manager performed an experiment to investigate the hydrolysis of 2-chlorooctane at different temperatures. The following results were obtained: Temperature (°C) Rate constant, k, (s-1) 1.06 x 10-5 3.19 x 10-4 9.86 x 10-4 2.92 x 10-3 25 35 45 Using a graphical method, calculate (i) the activation energy
A laboratory manager performed an experiment to investigate the hydrolysis of 2-chlorooctane at different temperatures. The following results were obtained: Temperature (°C) Rate constant, k, (s-1) 1.06 x 10-5 3.19 x 10-4 9.86 x 10-4 2.92 x 10-3 25 35 45 Using a graphical method, calculate (i) the activation energy
Chapter13: Kinetic Methods
Section: Chapter Questions
Problem 3P
Related questions
Question
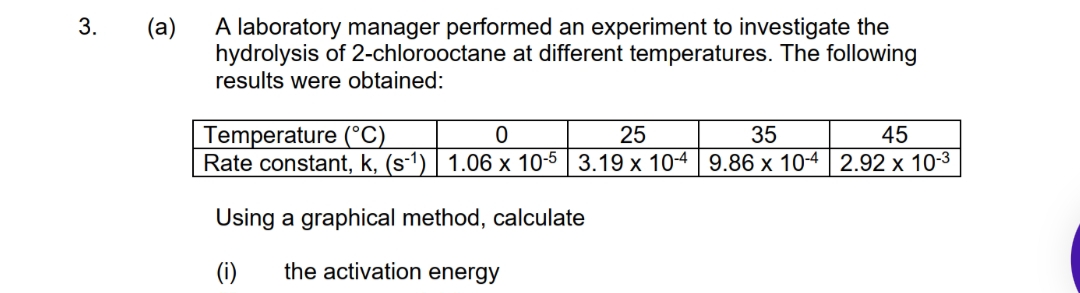
Transcribed Image Text:A laboratory manager performed an experiment to investigate the
hydrolysis of 2-chlorooctane at different temperatures. The following
results were obtained:
3.
(a)
Temperature (°C)
Rate constant, k, (s-1) | 1.06 x 10-5 3.19 x 10-4 9.86 x 10-4 2.92 x 10-3
25
35
45
Using a graphical method, calculate
(i)
the activation energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

