A large electrical power station generates 1050 MW of electricity with an efficiency of 37.0%. (a) Calculate the heat transfer (in J) to the power station, Q, in one day. (b) How much heat transfer Q, (in J) occurs to the environment in one day? (c) If the heat transfer in the cooling towers is from 35.0°C water into the local air mass, which increases in temperature from 18.0°C to 20.0°C, what is the total increase in entropy (in J/K) due to this heat transfer? J/K (d) How much energy (in J) becomes unavailable to do work because of this increase in entropy, assuming an 18.0°C lowest temperature? (Part of Q. could be utilized to operate heat engines or for simple space heating, but it rarely is.)
A large electrical power station generates 1050 MW of electricity with an efficiency of 37.0%. (a) Calculate the heat transfer (in J) to the power station, Q, in one day. (b) How much heat transfer Q, (in J) occurs to the environment in one day? (c) If the heat transfer in the cooling towers is from 35.0°C water into the local air mass, which increases in temperature from 18.0°C to 20.0°C, what is the total increase in entropy (in J/K) due to this heat transfer? J/K (d) How much energy (in J) becomes unavailable to do work because of this increase in entropy, assuming an 18.0°C lowest temperature? (Part of Q. could be utilized to operate heat engines or for simple space heating, but it rarely is.)
Chapter4: The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 77AP: A heat engine operates between two temperatures such that the working substance of the engine...
Related questions
Question
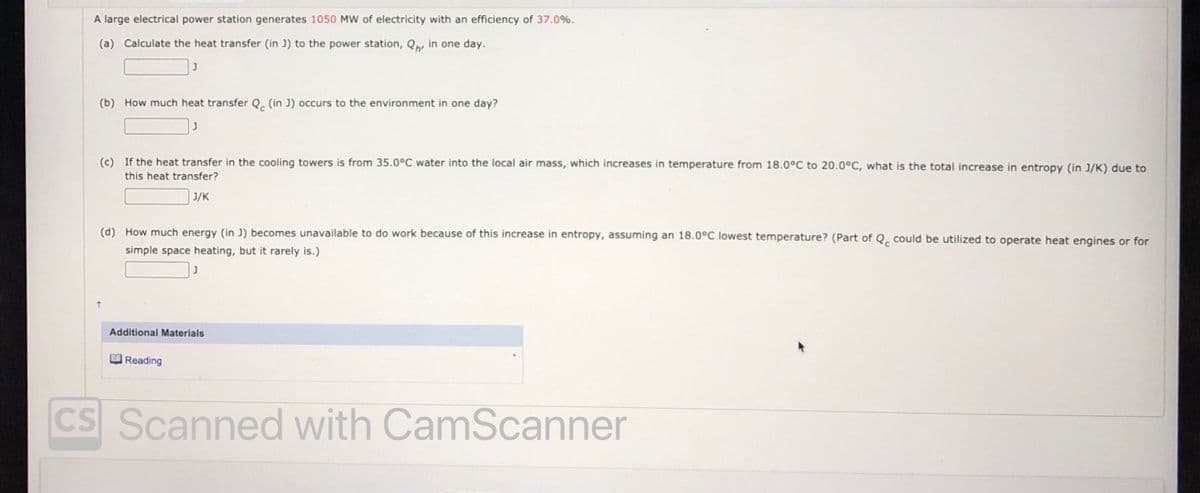
Transcribed Image Text:A large electrical power station generates 1050 MW of electricity with an efficiency of 37.0%.
(a) Calculate the heat transfer (in J) to the power station, Q, in one day.
(b) How much heat transfer Q. (in J) occurs to the environment in one day?
(c) If the heat transfer in the cooling towers is from 35.0°C water into the local air mass, which increases in temperature from 18.0°C to 20.0°C, what is the total increase in entropy (in J/K) due to
this heat transfer?
J/K
(d) How much energy (in J) becomes unavailable to do work because of this increase in entropy, assuming an 18.0°C lowest temperature? (Part of Q. could be utilized to operate heat engines or for
simple space heating, but it rarely is.)
to
Additional Materials
O Reading
CS Scanned with CamScanner
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning