A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The cylinder is also submerged in a large insulated water bath. (See sketch at right.) 1 atm pressure piston cylinder From previous experiments, this chemical reaction is known to release 124. kJ of water bath energy. gases alo The temperature of the water bath is monitored, and it is determined from this data that 354. kJ of heat flows into the system during the reaction. Ar |exothermic Is the reaction exothermic or endothermic? endothermic up Does the temperature of the water bath go up or down? down neither in Does the piston move in or out? out neither does work Does the gas mixture do work, or is work done on it? work is done on it neither How much work is done on (or by) the gas mixture? Be sure your answer has the correct number of significant digits.
A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The cylinder is also submerged in a large insulated water bath. (See sketch at right.) 1 atm pressure piston cylinder From previous experiments, this chemical reaction is known to release 124. kJ of water bath energy. gases alo The temperature of the water bath is monitored, and it is determined from this data that 354. kJ of heat flows into the system during the reaction. Ar |exothermic Is the reaction exothermic or endothermic? endothermic up Does the temperature of the water bath go up or down? down neither in Does the piston move in or out? out neither does work Does the gas mixture do work, or is work done on it? work is done on it neither How much work is done on (or by) the gas mixture? Be sure your answer has the correct number of significant digits.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.97PAE: 97 Homes in rural areas where natural gas service is not available often rely on propane to fuel...
Related questions
Question
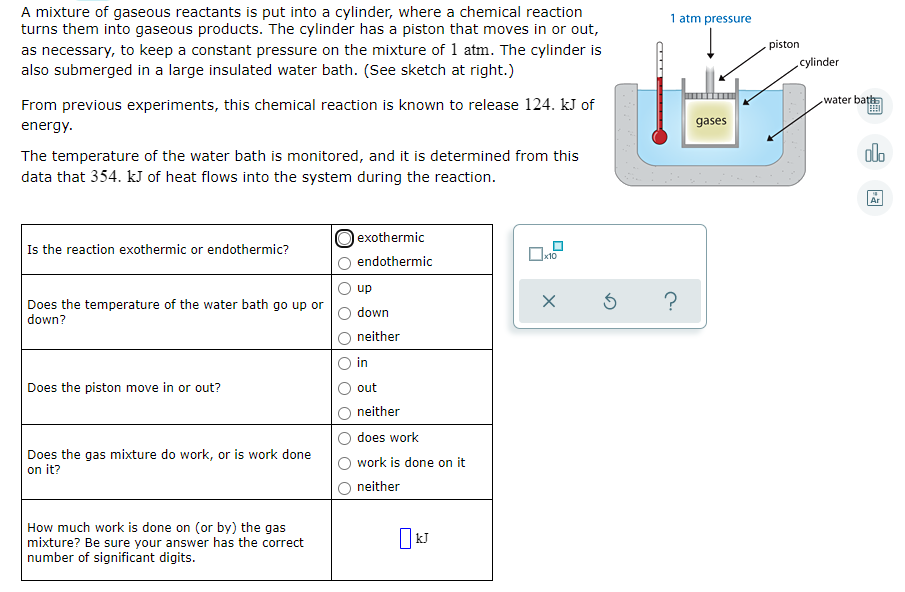
Transcribed Image Text:A mixture of gaseous reactants is put into a cylinder, where a chemical reaction
turns them into gaseous products. The cylinder has a piston that moves in or out,
as necessary, to keep a constant pressure on the mixture of 1 atm. The cylinder is
also submerged in a large insulated water bath. (See sketch at right.)
1 atm pressure
piston
cylinder
From previous experiments, this chemical reaction is known to release 124. kJ of
water bath
energy.
gases
dlo
The temperature of the water bath is monitored, and it is determined from this
data that 354. kJ of heat flows into the system during the reaction.
Ar
|exothermic
Is the reaction exothermic or endothermic?
endothermic
up
Does the temperature of the water bath go up or
down?
down
neither
in
Does the piston move in or out?
out
neither
does work
Does the gas mixture do work, or is work done
on it?
work is done on it
neither
How much work is done on (or by) the gas
mixture? Be sure your answer has the correct
number of significant digits.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning