A newly discovered element named cameroonium has the symbol Cm. What is oxidation number of cameroonium in the acid H3CMO3? O a. 2 O b. 6 O c. 4 O d. 1 О е. 3 O f. 5 A newly discovered element called mexicoum has the symbol Mx. Mexicoum is found in the following anions: Mx" mexicide MxO3 mexicite MxO4 mexicate What is the formula of mexicous acid? О а. НЗМх O b. H3MXO4 О с. НМхОд O d. HMXO3 О е. НаМхОд O f. HMx O g. H2MXO3
A newly discovered element named cameroonium has the symbol Cm. What is oxidation number of cameroonium in the acid H3CMO3? O a. 2 O b. 6 O c. 4 O d. 1 О е. 3 O f. 5 A newly discovered element called mexicoum has the symbol Mx. Mexicoum is found in the following anions: Mx" mexicide MxO3 mexicite MxO4 mexicate What is the formula of mexicous acid? О а. НЗМх O b. H3MXO4 О с. НМхОд O d. HMXO3 О е. НаМхОд O f. HMx O g. H2MXO3
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter18: Representative Metals, Metalloids, And Nonmetals
Section: Chapter Questions
Problem 84E: Write balanced chemical equations for the following reactions: (a) metallic aluminum burned in air...
Related questions
Question
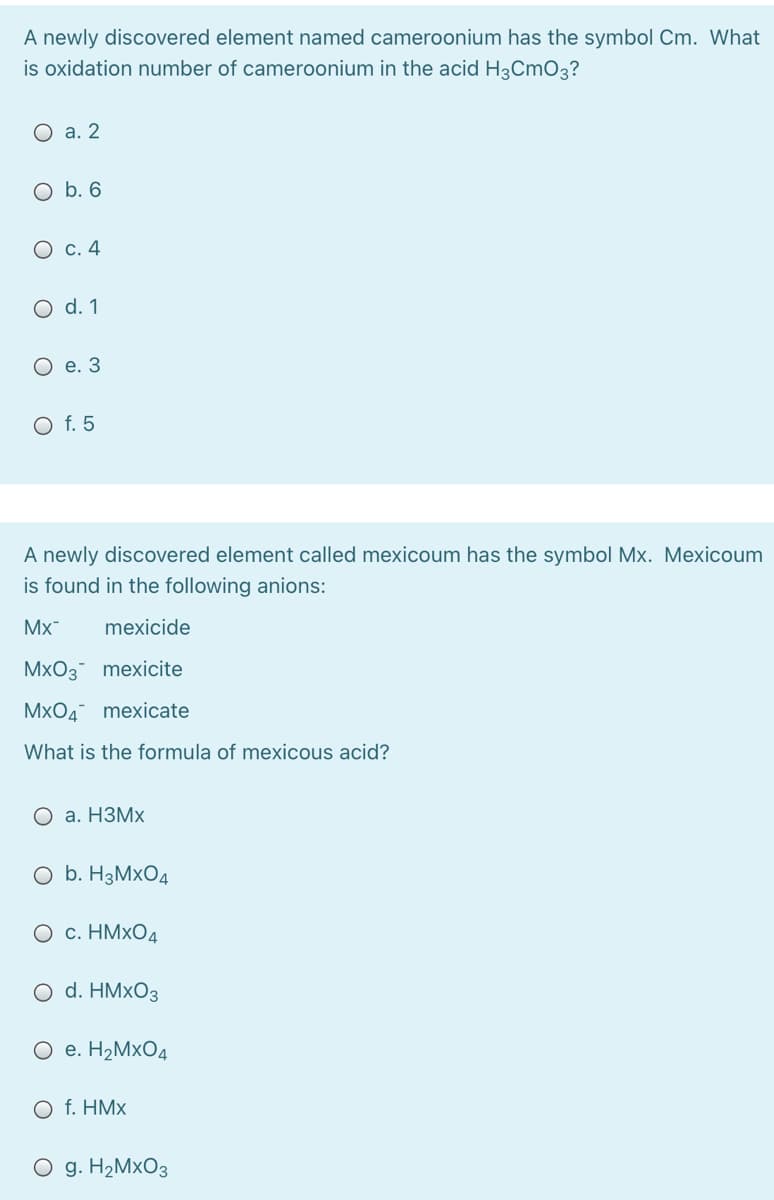
Transcribed Image Text:A newly discovered element named cameroonium has the symbol Cm. What
is oxidation number of cameroonium in the acid H3CMO3?
О а. 2
O b. 6
О с. 4
O d. 1
O e. 3
O f. 5
A newly discovered element called mexicoum has the symbol Mx. Mexicoum
is found in the following anions:
Mx
mexicide
MxO3 mexicite
MxO4 mexicate
What is the formula of mexicous acid?
О а. НЗМх
O b. H3M×O4
О с. НМхОд
O d. HM×O3
O e. H2M×O4
O f. HMx
O g. H2M×O3
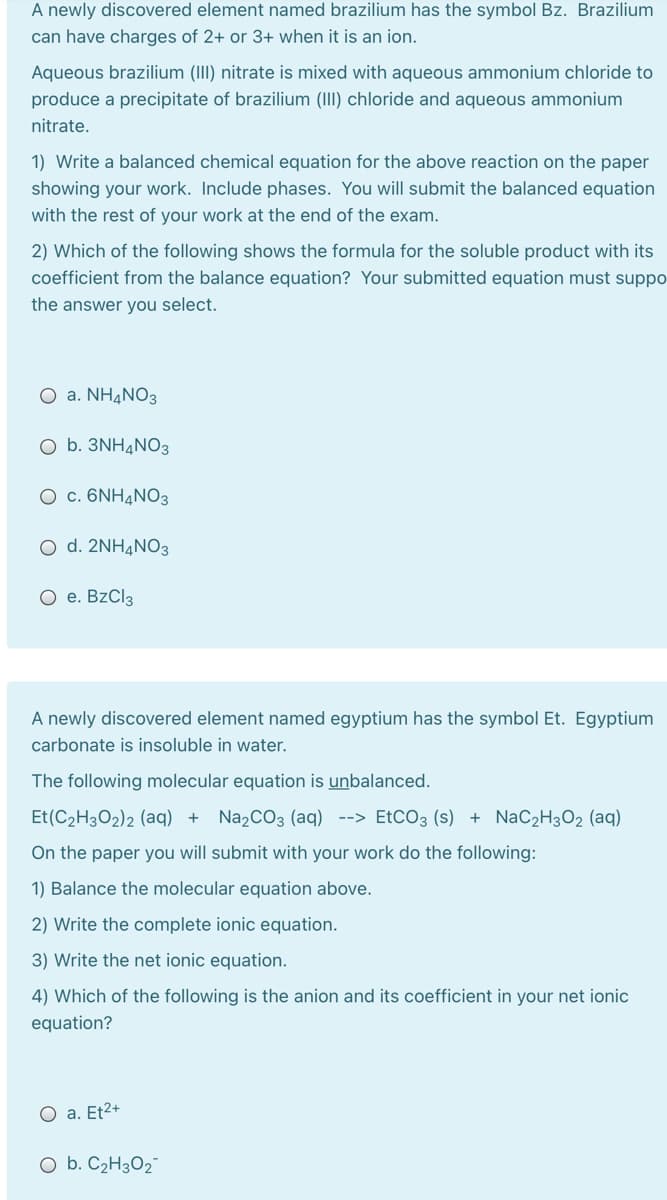
Transcribed Image Text:A newly discovered element named brazilium has the symbol Bz. Brazilium
can have charges of 2+ or 3+ when it is an ion.
Aqueous brazilium (III) nitrate is mixed with aqueous ammonium chloride to
produce a precipitate of brazilium (III) chloride and aqueous ammonium
nitrate.
1) Write a balanced chemical equation for the above reaction on the paper
showing your work. Include phases. You will submit the balanced equation
with the rest of your work at the end of the exam.
2) Which of the following shows the formula for the soluble product with its
coefficient from the balance equation? Your submitted equation must suppo-
the answer you select.
O a. NH¼NO3
O b. 3NH4NO3
O c. 6NH4NO3
d. 2NH4NO3
O e. BzCl3
A newly discovered element named egyptium has the symbol Et. Egyptium
carbonate is insoluble in water.
The following molecular equation is unbalanced.
Et(C2H3O2)2 (aq) +
Na2CO3 (aq) --> EtCO3 (s) + NaC2H3O2 (aq)
On the paper you will submit with your work do the following:
1) Balance the molecular equation above.
2) Write the complete ionic equation.
3) Write the net ionic equation.
4) Which of the following is the anion and its coefficient in your net ionic
equation?
O a. Et2+
O b. C2H3O2"
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning