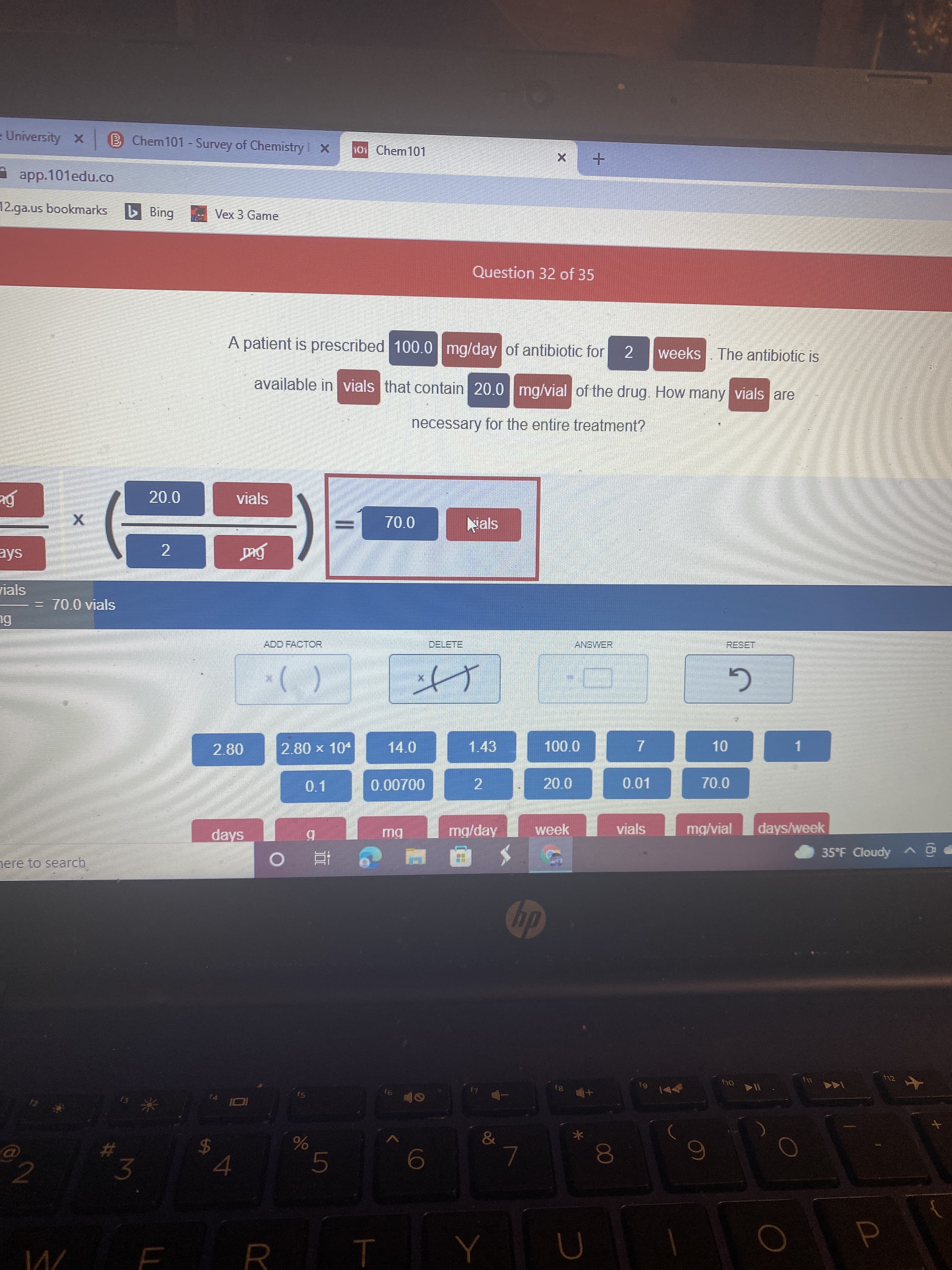
A patient is prescribed 100.0 mg/day of antibiotic for weeks. The antibiotic is available in vials that contain 20.0 mg/vial of the drug. How many vials are necessary for the entire treatment?
A patient is prescribed 100.0 mg/day of antibiotic for weeks. The antibiotic is available in vials that contain 20.0 mg/vial of the drug. How many vials are necessary for the entire treatment?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 56QAP: Magnesium chloride is an important coagulant used in the preparation of tofu from soy milk. Its...
Related questions
Question
Transcribed Image Text:00
2.
5
University X B Chem101 - Survey of Chemistry X
101 Chem101
app.101edu.co
12.ga.us bookmarks
L Bing
Vex 3 Game
Question 32 of 35
A patient is prescribed 100.0 mg/day of antibiotic for
weeks The antibiotic is
2.
available in vials that contain 20.0 mg/vial of the drug. How many vials are
necessary for the entire treatment?
vials
Nals
ays
2
ials
= 70.0 vials
ADD FACTOR
DELETE
ANSWER
RESET
( )
2.80
2.80 x 10
14.0
1.43
0 00
1.
7.
0.1
0.01
00L00'0
mg/day
week
vials
mg/vial
days/week
days
35°F Cloudy a @
nere to search
dy
f8
19 144
fs
91
&
24
4
3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning