A person makes a quantity of iced tea by mixing 480 g of hot tea (essentially water) with an equal mass of ice at its melting point. Assume the mixture has negligible energy exchanges with its environment. (a) If the tea's initial temperature is T; = 82°C, when thermal equilibrium is reached what are the mixture's temperature Tand (b) the remaining mass mfof ice? If T; = 57°C, when thermal equilibrium is reached what are (c) Tand (d) m? The specific heat of water is 4186 J/kg-K. The latent heat of fusion is 333 kJ/kg. (a) Number i Units (b) Number i Units (c) Number i Units (d) Number i Units
A person makes a quantity of iced tea by mixing 480 g of hot tea (essentially water) with an equal mass of ice at its melting point. Assume the mixture has negligible energy exchanges with its environment. (a) If the tea's initial temperature is T; = 82°C, when thermal equilibrium is reached what are the mixture's temperature Tand (b) the remaining mass mfof ice? If T; = 57°C, when thermal equilibrium is reached what are (c) Tand (d) m? The specific heat of water is 4186 J/kg-K. The latent heat of fusion is 333 kJ/kg. (a) Number i Units (b) Number i Units (c) Number i Units (d) Number i Units
Chapter1: Temperature And Heat
Section: Chapter Questions
Problem 80P: How many grams of coffee must evaporate from 350 g of coffee in a 100-g glass cup to cool the coffee...
Related questions
Question
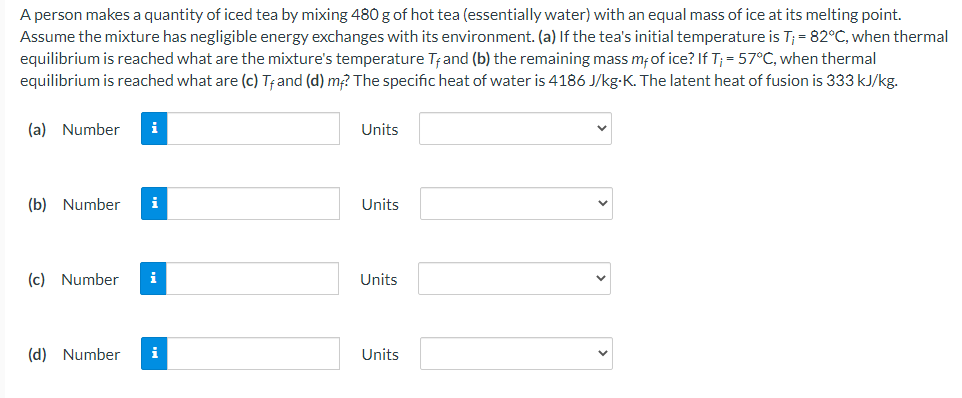
Transcribed Image Text:A person makes a quantity of iced tea by mixing 480 g of hot tea (essentially water) with an equal mass of ice at its melting point.
Assume the mixture has negligible energy exchanges with its environment. (a) If the tea's initial temperature is T; = 82°C, when thermal
equilibrium is reached what are the mixture's temperature Tand (b) the remaining mass mfof ice? If T; = 57°C, when thermal
equilibrium is reached what are (c) Tand (d) m? The specific heat of water is 4186 J/kg-K. The latent heat of fusion is 333 kJ/kg.
(a) Number
i
Units
(b) Number
i
Units
(c) Number
i
Units
(d) Number
i
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

