A piston-cylinder assembly contains air at a temperature of 527°C and a volume of V1 = 2 m³. The air undergoes three processes to complete a thermodynamic cycle. The following information is known about the three steps that make up the cycle: Process 1-2: Isothermal process from P = 436 kPa to P2 = 2300 kPa Process 2-3: Isobaric process until V1/V3 = 4. Process 3-1: Polytropic process to the initial state Plot the processes on the p-v diagram and Determine: (a) The polytropic exponent for process 3-1. (b) The change in the internal energy of air for each process. (c) The work done, and heat transfer for each process. (d) The net work, and net heat transferred during the cycle.
A piston-cylinder assembly contains air at a temperature of 527°C and a volume of V1 = 2 m³. The air undergoes three processes to complete a thermodynamic cycle. The following information is known about the three steps that make up the cycle: Process 1-2: Isothermal process from P = 436 kPa to P2 = 2300 kPa Process 2-3: Isobaric process until V1/V3 = 4. Process 3-1: Polytropic process to the initial state Plot the processes on the p-v diagram and Determine: (a) The polytropic exponent for process 3-1. (b) The change in the internal energy of air for each process. (c) The work done, and heat transfer for each process. (d) The net work, and net heat transferred during the cycle.
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter2: Matter And Energy
Section: Chapter Questions
Problem 27RQ: How many Btu/h would be produced in a 12-kW electricheater?
Related questions
Question
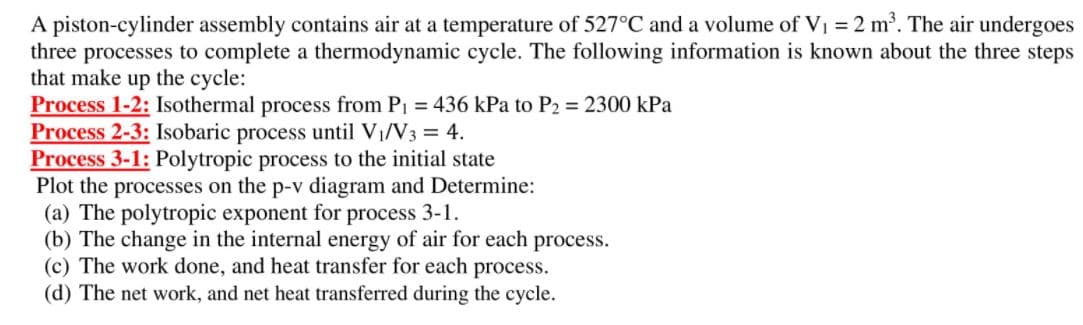
Transcribed Image Text:A piston-cylinder assembly contains air at a temperature of 527°C and a volume of Vi = 2 m. The air undergoes
three processes to complete a thermodynamic cycle. The following information is known about the three steps
that make up the cycle:
Process 1-2: Isothermal process from P1 = 436 kPa to P2 = 2300 kPa
Process 2-3: Isobaric process until VI/V3 = 4.
Process 3-1: Polytropic process to the initial state
Plot the processes on the p-v diagram and Determine:
(a) The polytropic exponent for process 3-1.
(b) The change in the internal energy of air for each process.
(c) The work done, and heat transfer for each process.
(d) The net work, and net heat transferred during the cycle.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning