A piston-cylinder assembly contains water that undergoes a series of processes. Process 1-->2: Constant-volume heating from p1=5bar and T1-160° to P2=10bar Process 2-->3: Constant-pressure cooling to saturated vapor Process 3-->4: Constant-volume cooling to T4=160°C Process 4-->5: Constant-temperature expansion with Q=815.8 kJ 1- Sketch the processes on Pt, P, and Tv, plots as the following Label the axes PRESSURE, TEMPERATURE and SPECIFIC VOLUME values; use closed dots to show the states, use solid lines to connect the states, add number and arrows to make clear the states numbers and process directions. 2- Could this be considered a thermodynamic cycle? Why or why not?
A piston-cylinder assembly contains water that undergoes a series of processes. Process 1-->2: Constant-volume heating from p1=5bar and T1-160° to P2=10bar Process 2-->3: Constant-pressure cooling to saturated vapor Process 3-->4: Constant-volume cooling to T4=160°C Process 4-->5: Constant-temperature expansion with Q=815.8 kJ 1- Sketch the processes on Pt, P, and Tv, plots as the following Label the axes PRESSURE, TEMPERATURE and SPECIFIC VOLUME values; use closed dots to show the states, use solid lines to connect the states, add number and arrows to make clear the states numbers and process directions. 2- Could this be considered a thermodynamic cycle? Why or why not?
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter1: Heat, Temperature, And Pressure
Section: Chapter Questions
Problem 6RQ: One British thermal unit will raise the temperature of _____ 1b of water _____F.
Related questions
Question
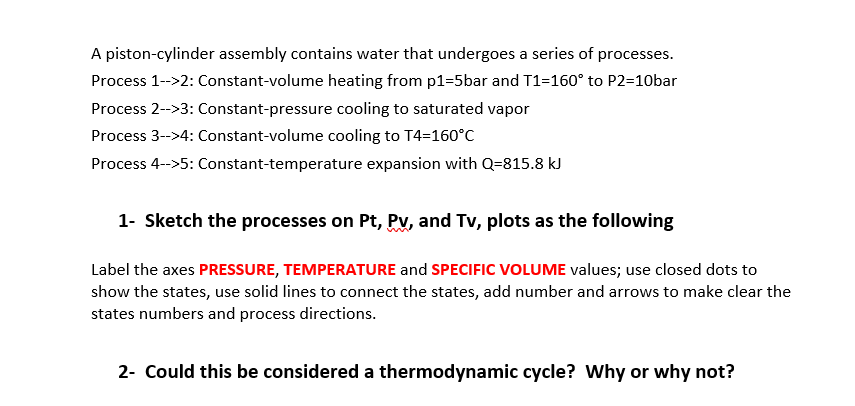
Transcribed Image Text:A piston-cylinder assembly contains water that undergoes a series of processes.
Process 1-->2: Constant-volume heating from p1=5bar and T1=160° to P2=10bar
Process 2-->3: Constant-pressure cooling to saturated vapor
Process 3-->4: Constant-volume cooling to T4=160°C
Process 4-->5: Constant-temperature expansion with Q=815.8 kJ
1- Sketch the processes on Pt, Pyv, and Tv, plots as the following
Label the axes PRESSURE, TEMPERATURE and SPECIFIC VvOLUME values; use closed dots to
show the states, use solid lines to connect the states, add number and arrows to make clear the
states numbers and process directions.
2- Could this be considered a thermodynamic cycle? Why or why not?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning