A prepared buffer solution contains a mixture of H2PO4 and HPO ions. Which species will react with any H+ ions added to the solution? H3O+ • H₂PO OH ○ HPO2- ΗΡΟ
A prepared buffer solution contains a mixture of H2PO4 and HPO ions. Which species will react with any H+ ions added to the solution? H3O+ • H₂PO OH ○ HPO2- ΗΡΟ
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter14: Acid-base Equilibria
Section: Chapter Questions
Problem 97E: What will be the pH of a buffer solution prepared from 0.20 mol NH3, 0.40 mol NH4NO3, and just...
Related questions
Question
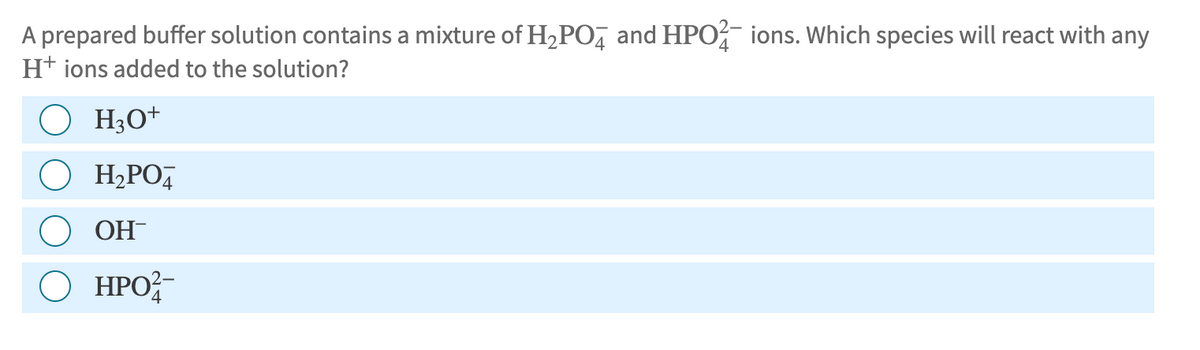
Transcribed Image Text:A prepared buffer solution contains a mixture of H2PO4 and HPO ions. Which species will react with any
H+ ions added to the solution?
H3O+
• H₂PO
OH
○ HPO2-
ΗΡΟ
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning