A process by which zirconium metal can be produced from the mineral zirconium (IV) orthosilicate, ZrSiO4, starts by reacting it with chlorine gas to form zirconium (IV) chloride. ZrSiO4 + 2 Cl2 → ZrCl4 + S¡O2 + O2 If 862 g ZrSiO4 and 950. g of Cl, are available, what is the limiting reactant (blank 1, no subscripts needed)? What is the mass of ZrCl4 (blank 2, no decimal places, rounded to the nearest 100)?
A process by which zirconium metal can be produced from the mineral zirconium (IV) orthosilicate, ZrSiO4, starts by reacting it with chlorine gas to form zirconium (IV) chloride. ZrSiO4 + 2 Cl2 → ZrCl4 + S¡O2 + O2 If 862 g ZrSiO4 and 950. g of Cl, are available, what is the limiting reactant (blank 1, no subscripts needed)? What is the mass of ZrCl4 (blank 2, no decimal places, rounded to the nearest 100)?
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter3: Calculations With Chemical Formulas And Equaitons
Section: Chapter Questions
Problem 3.20QP: Propane, C3H8, is the fuel of choice in a gas barbecue. When burning, the balanced equation is...
Related questions
Question
100%
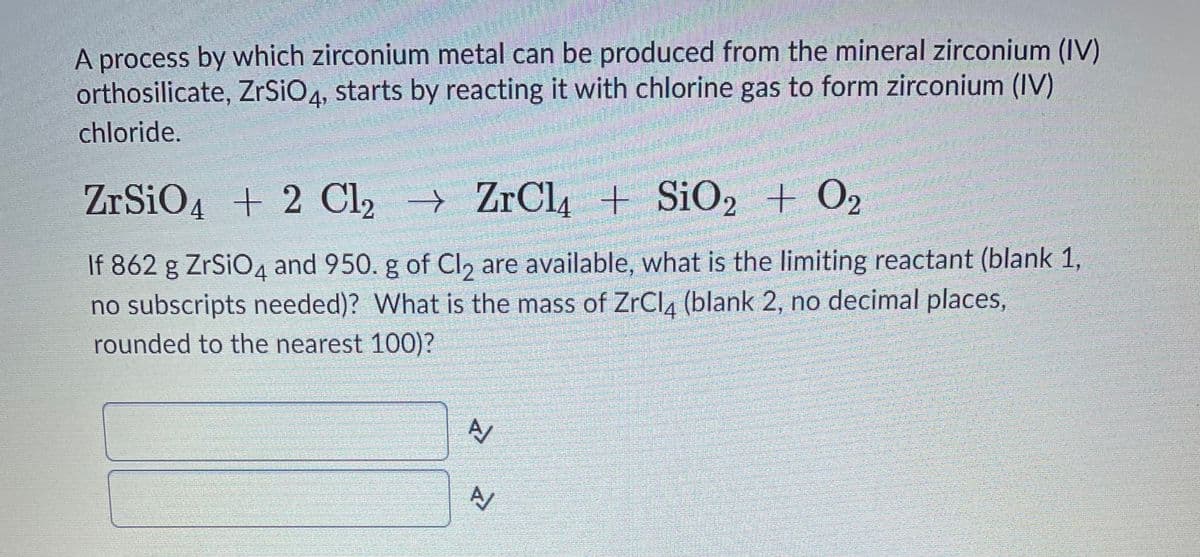
Transcribed Image Text:A process by which zirconium metal can be produced from the mineral zirconium (IV)
orthosilicate, ZrSiO4, starts by reacting it with chlorine gas to form zirconium (IV)
chloride.
ZrSiO4 + 2 Cl, ZrCl4 + SiO, + O2
If 862 g ZrSiO4 and 950. g of Cl, are available, what is the limiting reactant (blank 1,
no subscripts needed)? What is the mass of ZrCla (blank 2, no decimal places,
rounded to the nearest 100)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div