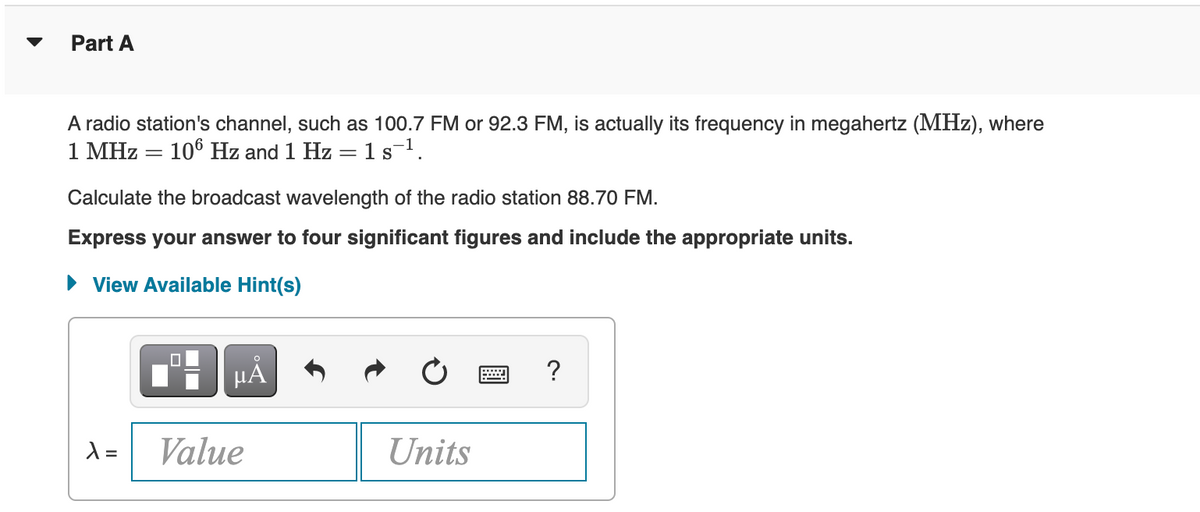
A radio station's channel, such as 100.7 FM or 92.3 FM, is actually its frequency in megahertz (MHz), where 1 MHz = 106 Hz and 1 Hz = 1 s¯¹. Calculate the broadcast wavelength of the radio station 88.70 FM. Express your answer to four significant figures and include the appropriate units. ► View Available Hint(s)
A radio station's channel, such as 100.7 FM or 92.3 FM, is actually its frequency in megahertz (MHz), where 1 MHz = 106 Hz and 1 Hz = 1 s¯¹. Calculate the broadcast wavelength of the radio station 88.70 FM. Express your answer to four significant figures and include the appropriate units. ► View Available Hint(s)
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter9: Nuclear Chemistry
Section: Chapter Questions
Problem 9.10P: 9-10 Microwaves are a form of electromagnetic radiation that is used for the rapid heating of foods....
Related questions
Question
Pls help ASAP. Pls do both of the asked parts.
Transcribed Image Text:Part A
A radio station's channel, such as 100.7 FM or 92.3 FM, is actually its frequency in megahertz (MHz), where
1 MHz = 106 Hz and 1 Hz = 1 s¯¹.
Calculate the broadcast wavelength of the radio station 88.70 FM.
Express your answer to four significant figures and include the appropriate units.
► View Available Hint(s)
µA
λ =
Value
Units
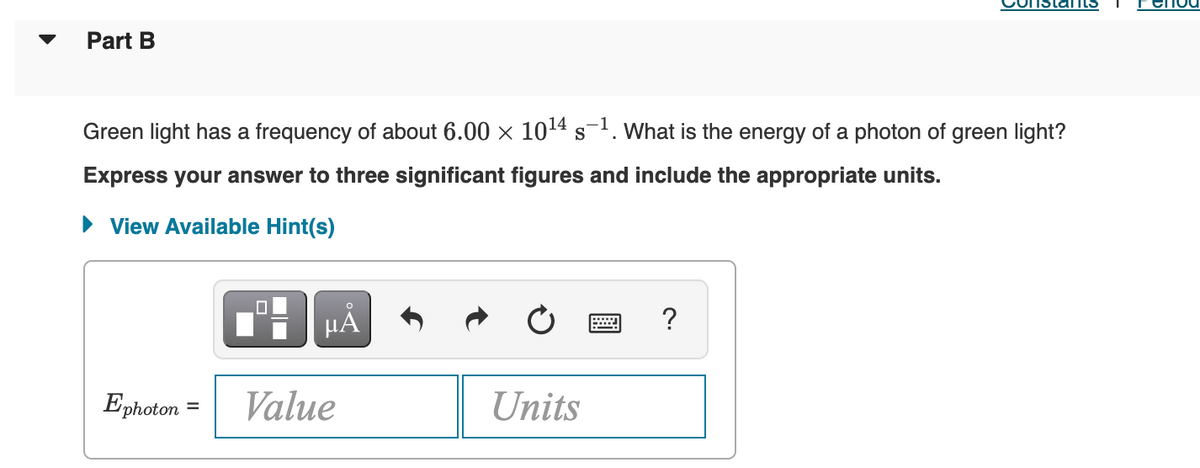
Transcribed Image Text:Part B
Green light has a frequency of about 6.00 × 10¹4 s-¹. What is the energy of a photon of green light?
Express your answer to three significant figures and include the appropriate units.
View Available Hint(s)
?
Ephoton Value
Units
µÅ
Cernoo
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning