A sample containing NaCl, Na,SO, and NaNO, gives the following elemental analysis: Na: 33.807 percent; O: 37.01 percent; Cl: 16.67 percent. Calculate the mass percent of each compound in the sample. % NaCl % NazSO, % NaNO3
A sample containing NaCl, Na,SO, and NaNO, gives the following elemental analysis: Na: 33.807 percent; O: 37.01 percent; Cl: 16.67 percent. Calculate the mass percent of each compound in the sample. % NaCl % NazSO, % NaNO3
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 154CWP: Arrange the following substances in order of increasing mass percent of nitrogen. a. NO b. N2O c....
Related questions
Question
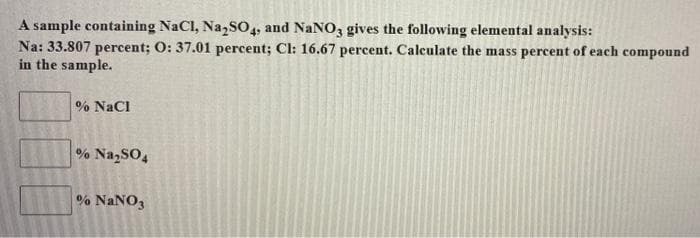
Transcribed Image Text:A sample containing NaCl, Na,SO,, and NaNO, gives the following elemental analysis:
Na: 33.807 percent; O: 37.01 percent; Cl: 16.67 percent. Calculate the mass percent of each compound
in the sample.
% NaCl
% NazSO4
% NaNO3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning