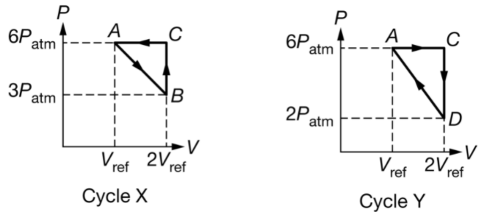
A sample of an ideal gas can be taken through the two cycles shown in the graphs of pressure Pas a function of volume V. In each cycle the gas starts and ends in state A. The gas is contained in a cylindrical container with a movable piston. Rank the temperature T of the gas for each labeled state in cycle X. Clearly indicate the order of ranking and if any states have the same temperature. Justify your ranking using information from the graph.
A sample of an ideal gas can be taken through the two cycles shown in the graphs of pressure Pas a function of volume V. In each cycle the gas starts and ends in state A. The gas is contained in a cylindrical container with a movable piston. Rank the temperature T of the gas for each labeled state in cycle X. Clearly indicate the order of ranking and if any states have the same temperature. Justify your ranking using information from the graph.
Chapter3: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 4CQ: Why does a bicycle pump feel warm as you inflate your tire?
Related questions
Question
A sample of an ideal gas can be taken through the two cycles shown in the graphs of pressure Pas a function of volume V. In each cycle the gas starts and ends in state A. The gas is contained in a cylindrical container with a movable piston. Rank the temperature T of the gas for each labeled state in cycle X. Clearly indicate the order of ranking and if any states have the same temperature. Justify your ranking using information from the graph.
Transcribed Image Text:P
P
6P
A
6P.
atm
A
atm
3P
atm
2P.
atm
2V
Vref
Vref 2V
ref
ref
Сycle X
Cycle Y
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University