A sample of an unknown protein dissolved in water to give 25.0 mL of solution was found to have an osmotic pressure of 3.22 torr at 25°C. What was the concentration of the protein in the sample in units of molarity. A) 1.73 x 10 M B) 5.77 x 10 M C) 1.71 x 10 M D) 0.132 M E) 0.0155 M
A sample of an unknown protein dissolved in water to give 25.0 mL of solution was found to have an osmotic pressure of 3.22 torr at 25°C. What was the concentration of the protein in the sample in units of molarity. A) 1.73 x 10 M B) 5.77 x 10 M C) 1.71 x 10 M D) 0.132 M E) 0.0155 M
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.89QE
Related questions
Question
Transcribed Image Text:nos | Scientific Calculator
yam/Downloads/CBC%20CHEM162%20W21%20Quiz4.pdf
O B Page view
ARead aloud V Draw
Highlic
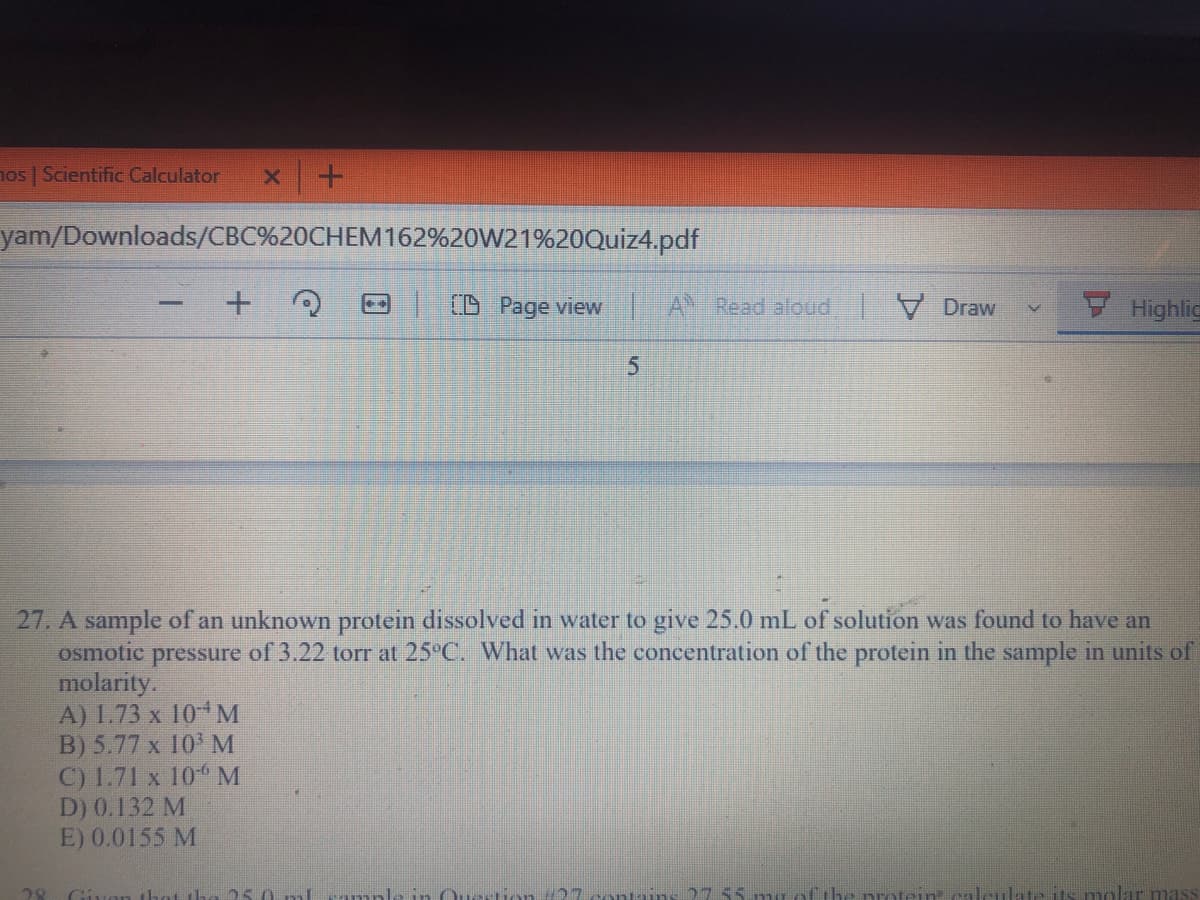
27. A sample of an unknown protein dissolved in water to give 25.0 mL of solution was found to have an
of 3.22 torr at 25°C. What was the concentration of the protein in the sample in units of
osmotic
pressure
molarity.
A) 1.73 x 104M
B) 5.77 x 10 M
C) 1.71 x 10 M
D) 0.132 M
E) 0.0155 M
28 Ciuan that h
25.0 ml
55.mu o
molar mass
Expert Solution
Step 1
Given : Volume of solution = 25.0 mL = 0.0250 L (since 1 L = 1000 mL)
Osmotic pressure = 3.22 torr = 3.22 / 760 = 4.237 X 10-3 atm (since 1 atm = 760 torr)
And temperature = 25 oC
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
