A sample of lead isotope Pb decays by emitting an alpha particle to form the nuclide W. If the half- life of the lead isotope is 3 hours, which of the following statements is/are correct? 1. W has an atomic mass of 205 2. The atomic number of W is 80 3. After 6 hours, 50% of the lead isotope would have decayed. 40. A B
A sample of lead isotope Pb decays by emitting an alpha particle to form the nuclide W. If the half- life of the lead isotope is 3 hours, which of the following statements is/are correct? 1. W has an atomic mass of 205 2. The atomic number of W is 80 3. After 6 hours, 50% of the lead isotope would have decayed. 40. A B
Chapter5: Stereochemistry At Tetrahedral Centers
Section5.SE: Something Extra
Problem 58AP: One of the steps in fat metabolism is the hydration of crotonate to yield 3-hydroxybutyrate. This...
Related questions
Question
show-all-working-explaining-detailly-each-step.
Answer should be typewritten using a computer keyboard.

Transcribed Image Text:A sample of lead isotope Pb decays by
emitting an alpha particle to form the nuclide
W. If the half- life of the lead isotope is 3
hours, which of the following statements
is/are correct?
209
40.
82
1. W has an atomic mass of 205
2. The atomic number of W is 80
3. After 6 hours, 50% of the lead isotope
would have decayed.
A.
BCD
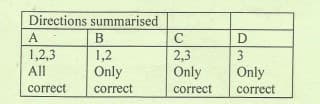
Transcribed Image Text:Directions summarised
A
B
1,2,3
1,2
2,3
Only
All
Only
Only
correct
correct
correct
correct
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

