a) Sodium carbonate, Na2CO3 (105.9888 g/mol) has been widely applied in many chemical processes such as manufacture of detergents, soaps, paper. Simon prepared a 10 % w/w sodium hydroxide solution with density of 1.1029 g/mL using a 1 L volumetric flask. Deduce the molarity of sodium hydroxide solution that Simon has prepared.
a) Sodium carbonate, Na2CO3 (105.9888 g/mol) has been widely applied in many chemical processes such as manufacture of detergents, soaps, paper. Simon prepared a 10 % w/w sodium hydroxide solution with density of 1.1029 g/mL using a 1 L volumetric flask. Deduce the molarity of sodium hydroxide solution that Simon has prepared.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section: Chapter Questions
Problem 57GQ: Dimethylglyoxime [DMG, (CH3CNOH)2] is used as a reagent to precipitate nickel ion. Assume that 53.0...
Related questions
Question
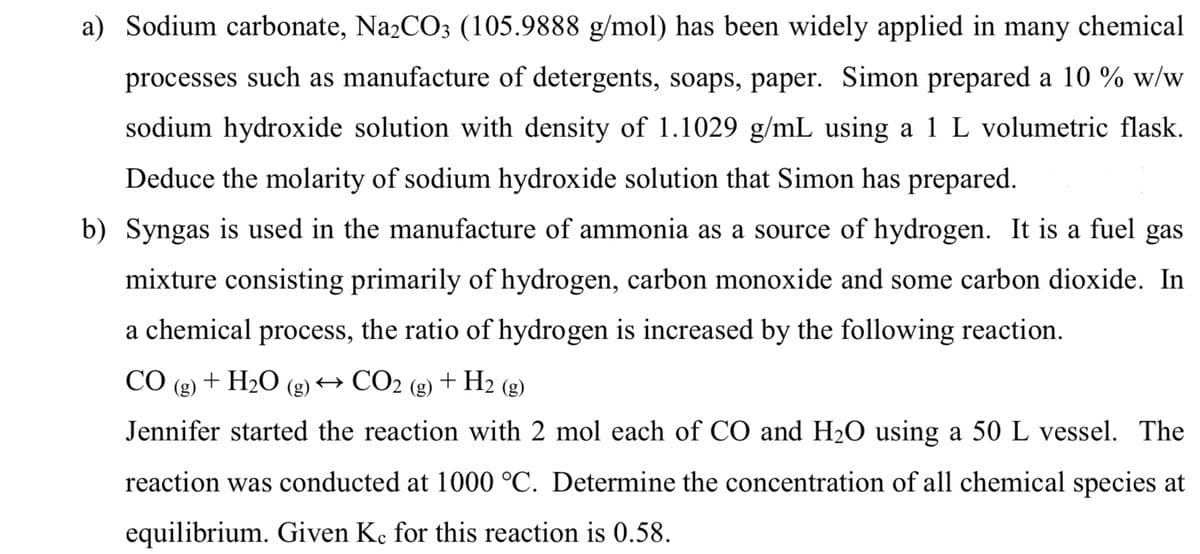
Transcribed Image Text:a) Sodium carbonate, Na₂CO3 (105.9888 g/mol) has been widely applied in many chemical
processes such as manufacture of detergents, soaps, paper. Simon prepared a 10 % w/w
sodium hydroxide solution with density of 1.1029 g/mL using a 1 L volumetric flask.
Deduce the molarity of sodium hydroxide solution that Simon has prepared.
b) Syngas is used in the manufacture of ammonia as a source of hydrogen. It is a fuel gas
mixture consisting primarily of hydrogen, carbon monoxide and some carbon dioxide. In
a chemical process, the ratio of hydrogen is increased by the following reaction.
CO (g) + H₂O
+ H₂ (g)
→ CO₂
(g)
(g)
Jennifer started the reaction with 2 mol each of CO and H₂O using a 50 L vessel. The
reaction was conducted at 1000 °C. Determine the concentration of all chemical species at
equilibrium. Given Ke for this reaction is 0.58.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning