A solid sample of strontium contains 2.27x1021 FCC unit cells. Determine the volume of the solid sample (in cm³) if Sr has a density of 2.64 g/cm³ and an atomic weight of 87.62 g/mol.
A solid sample of strontium contains 2.27x1021 FCC unit cells. Determine the volume of the solid sample (in cm³) if Sr has a density of 2.64 g/cm³ and an atomic weight of 87.62 g/mol.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter12: The Solid State
Section12.2: Structures And Formulas Of Ionic Solids
Problem 1RC: 1. The unit cell of silicon carbide. SiC is illustrated in the margin. In what type of unit cell are...
Related questions
Question
100%
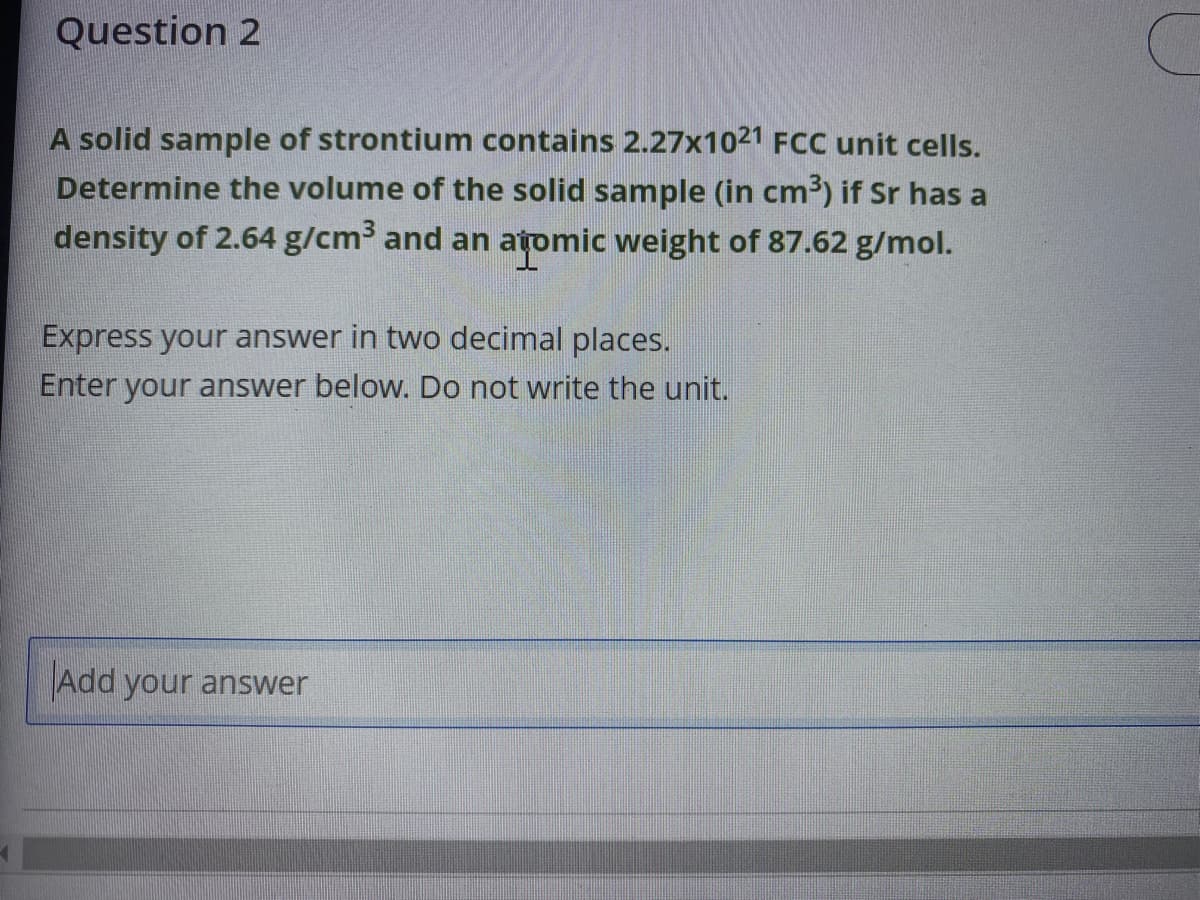
Transcribed Image Text:Question 2
A solid sample of strontium contains 2.27x1021 FCC unit cells.
Determine the volume of the solid sample (in cm³) if Sr has a
density of 2.64 g/cm³ and an atomic weight of 87.62 g/mol.
Express your answer in two decimal places.
Enter your answer below. Do not write the unit.
Add your answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
