A solution contains some or all of the ions Cu²+, Al³+, K, Ca²+, Ba²+, Pb²+, and NH₁. The following tests were performed, in order, on the solution. 1. Addition of 6 M HCl produced no reaction. 2. Addition of H₂S with 0.2 M HCl produced a black solid. 3. Addition of (NH4)2S produced a white solid. 4. Addition of (NH4)2HPO4 in NH, produced no reaction. 5. The final supernatant when heated produced a purple flame. Identify which of these ions were present in the solution, which were absent, and for which ones no conclusions can be drawn. Drag the appropriate items to their respective bins. ▸ View Available Hint(s) Present Cu²+ Pb²+ Ba²+ Absent. A1³+ K+ Ca²+ NH₂ Inconclusive Re
A solution contains some or all of the ions Cu²+, Al³+, K, Ca²+, Ba²+, Pb²+, and NH₁. The following tests were performed, in order, on the solution. 1. Addition of 6 M HCl produced no reaction. 2. Addition of H₂S with 0.2 M HCl produced a black solid. 3. Addition of (NH4)2S produced a white solid. 4. Addition of (NH4)2HPO4 in NH, produced no reaction. 5. The final supernatant when heated produced a purple flame. Identify which of these ions were present in the solution, which were absent, and for which ones no conclusions can be drawn. Drag the appropriate items to their respective bins. ▸ View Available Hint(s) Present Cu²+ Pb²+ Ba²+ Absent. A1³+ K+ Ca²+ NH₂ Inconclusive Re
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter15: Principles Of Chemical Reactivity: Equilibria
Section: Chapter Questions
Problem 66IL: The photograph below shows what occurs when a solution of potassium chromate is treated with a few...
Related questions
Question
2
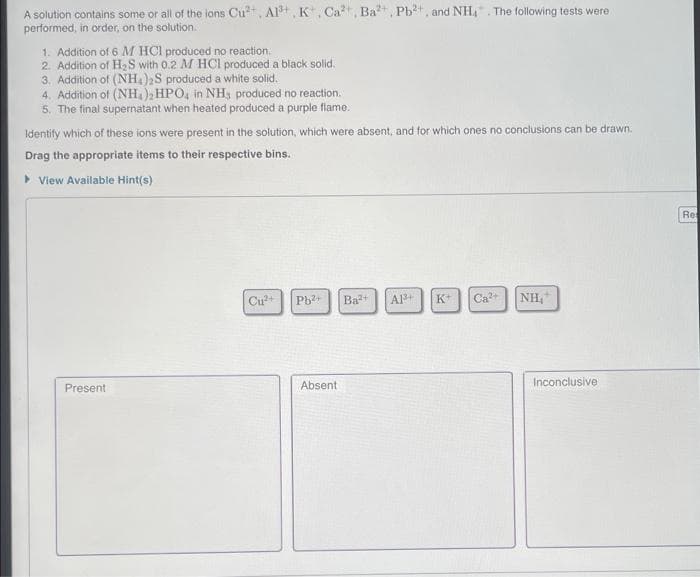
Transcribed Image Text:A solution contains some or all of the ions Cu²+, Al³+, K, Ca, Ba²+, Pb²+, and NH₂. The following tests were
performed, in order, on the solution.
1. Addition of 6 M HCl produced no reaction.
2. Addition of H₂S with 0.2 M HCl produced a black solid.
3. Addition of (NH4)2S produced a white solid.
4. Addition of (NH4)2HPO4 in NH, produced no reaction.
5. The final supernatant when heated produced a purple flame.
Identify which of these ions were present in the solution, which were absent, and for which ones no conclusions can be drawn.
Drag the appropriate items to their respective bins.
▸ View Available Hint(s)
Present
Cu²+ Pb²+ Ba²+ A1³+ K+ Ca²+ NH₁
Absent
Inconclusive
Re
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning