A solution is made of two volatile solutes: Chemical A (with a pure vapor pressure of 80.0 mm Hg) and Chemical B (with a pure vapor pressure of 100.0 mm Hg). The solution has a total vapor pressure of (9.66x10^1) mm Hg. If the solution is known to be made from 3.00 mol of A, how many moles of B must there be in the solution?
A solution is made of two volatile solutes: Chemical A (with a pure vapor pressure of 80.0 mm Hg) and Chemical B (with a pure vapor pressure of 100.0 mm Hg). The solution has a total vapor pressure of (9.66x10^1) mm Hg. If the solution is known to be made from 3.00 mol of A, how many moles of B must there be in the solution?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter13: The Chemistry Of Solutes And Solutions
Section: Chapter Questions
Problem 127QRT
Related questions
Question

Transcribed Image Text:A solution is made of two volatile solutes: Chemical A (with a pure vapor pressure of
80.0 mm Hg) and ChemicalB (with a pure vapor pressure of 100.0 mm Hg). The
solution has a total vapor pressure of (9.66x10^1) mm Hg.
If the solution is known to be made from 3.00 mol of A, how many moles of B must
there be in the solution?
HINT: The total pressure is the sum of each chemical's partial pressure: Pt = PA +
PB.
Use PA = XA PA° for both the partial pressure of chemicals A and B.
Enter your answer in scientific notation with 3 sig figs. Do not include any units in
your answer.
Do not round any intermediate calculations.
Note: Your answer is assumed to be reduced to the highest power possible.
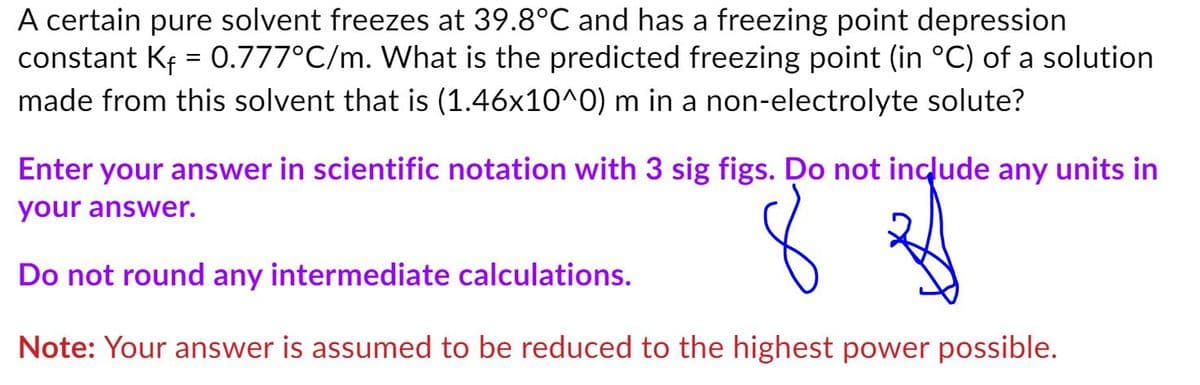
Transcribed Image Text:A certain pure solvent freezes at 39.8°C and has a freezing point depression
constant Kf = 0.777°C/m. What is the predicted freezing point (in °C) of a solution
made from this solvent that is (1.46x10^0) m in a non-electrolyte solute?
Enter your answer in scientific notation with 3 sig figs. Do not include any units in
your answer.
Do not round any intermediate calculations.
Note: Your answer is assumed to be reduced to the highest power possible.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning