A student analyzes a sample of rock that contains the element calcium (Ca). In the student's analysis, the calcium in the rock is precipitated as CaC,04 by the addition of Na,C,04. It is determined by the student that 182.2 mg of the rock produces 120.7 mg of CaC,04. What is the mass percent of calcium (Ca) in the rock sample? molar mass Ca = 40.078 g/mol molar mass CaC,04 = 128.097 g/mol molar mass Na, C,O4- 133.9985 g/mol
A student analyzes a sample of rock that contains the element calcium (Ca). In the student's analysis, the calcium in the rock is precipitated as CaC,04 by the addition of Na,C,04. It is determined by the student that 182.2 mg of the rock produces 120.7 mg of CaC,04. What is the mass percent of calcium (Ca) in the rock sample? molar mass Ca = 40.078 g/mol molar mass CaC,04 = 128.097 g/mol molar mass Na, C,O4- 133.9985 g/mol
Question
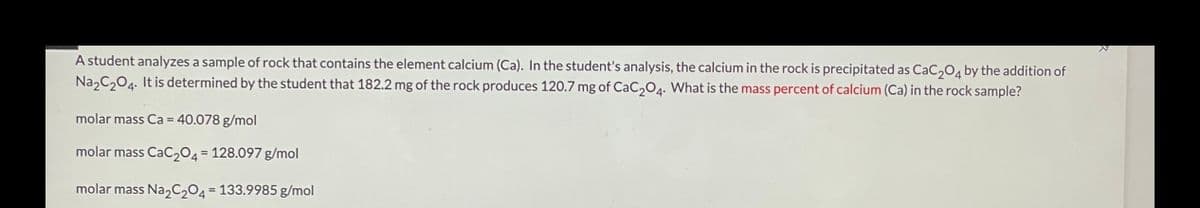
Transcribed Image Text:A student analyzes a sample of rock that contains the element calcium (Ca). In the student's analysis, the calcium in the rock is precipitated as CaC,04 by the addition of
Na,C204. It is determined by the student that 182.2 mg of the rock produces 120.7 mg of CaC,04. What is the mass percent of calcium (Ca) in the rock sample?
molar mass Ca = 40.078 g/mol
molar mass CaC204= 128.097 g/mol
molar mass Na2C204 = 133.9985 g/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.