A system consists of a particle of mass 5000.0 -20 x10 g confined to a one-dimensional box having a length of 4.0 x 10 m. Calculate the frequency in s that corresponds to the transition between the levels n=3 and n=5 of this system? 10 -34 (Plank constant (h) = 6.626 x 10JS) Select one: 72471.88 854365.02 124237.50 217415.63 165650.00
A system consists of a particle of mass 5000.0 -20 x10 g confined to a one-dimensional box having a length of 4.0 x 10 m. Calculate the frequency in s that corresponds to the transition between the levels n=3 and n=5 of this system? 10 -34 (Plank constant (h) = 6.626 x 10JS) Select one: 72471.88 854365.02 124237.50 217415.63 165650.00
Related questions
Question
B5
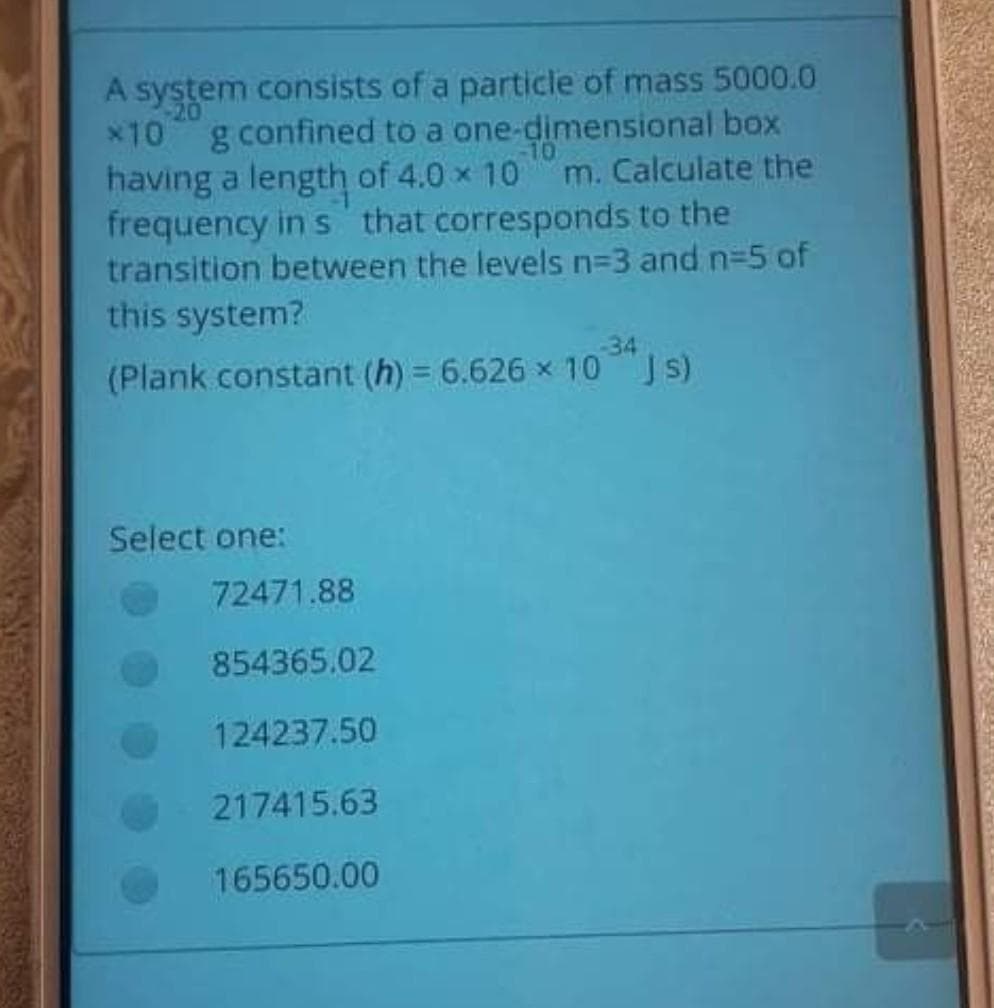
Transcribed Image Text:system consists of a particle of mass 5000.0
x10 g confined to a one-dimensional box
having a length of 4.0 x 10
frequency in s that corresponds to the
transition between the levels n=3 and n-5 of
this system?
10
m. Calculate the
34
(Plank constant (h) = 6.626 x 10 JS)
Select one:
72471.88
854365.02
124237.50
217415.63
165650.00

Transcribed Image Text:In the vibrational-rotational spectrum of a
certain molecule, the line corresponding to
the transition J"=4-J'=3 appear at 2600 cm
Calculate the position of the line
corresponding to the J"=1-J'=2 transition in
wavenumbers (cm )?
The rotational constant is equal to 10.0 cm?
(assume Av=+1, upper level having quantum
numbers v' and J' and a lower level with v"
and J")
Select one:
2780
2700
2740
2760
2720
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps
