(a) Take the definition of the coefficient of volume expan- sion to be 1 dv B = V dT 1 av V aT P=constant Use the equation of state for an ideal gas to show that the coefficient of volume expansion for an ideal gas at constant pressure is given by B = 1/T, where Tis the absolute temper- ature. (b) What value does this expression predict for B at 0°C? State how this result compares with the experimental values for (c) helium and (d) air in Table 18.1. Note: These values are much larger than the coefficients of volume expansion for most liquids and solids.
(a) Take the definition of the coefficient of volume expan- sion to be 1 dv B = V dT 1 av V aT P=constant Use the equation of state for an ideal gas to show that the coefficient of volume expansion for an ideal gas at constant pressure is given by B = 1/T, where Tis the absolute temper- ature. (b) What value does this expression predict for B at 0°C? State how this result compares with the experimental values for (c) helium and (d) air in Table 18.1. Note: These values are much larger than the coefficients of volume expansion for most liquids and solids.
Chapter2: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 70P: Using a numerical integration method such as Simpson's rule, find the fraction of molecules in a...
Related questions
Question
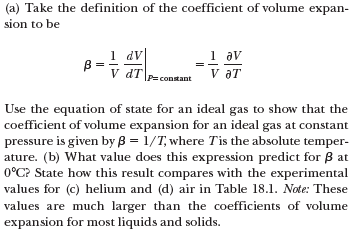
Transcribed Image Text:(a) Take the definition of the coefficient of volume expan-
sion to be
1 dv
B =
V dT
1 av
V aT
P=constant
Use the equation of state for an ideal gas to show that the
coefficient of volume expansion for an ideal gas at constant
pressure is given by B = 1/T, where Tis the absolute temper-
ature. (b) What value does this expression predict for B at
0°C? State how this result compares with the experimental
values for (c) helium and (d) air in Table 18.1. Note: These
values are much larger than the coefficients of volume
expansion for most liquids and solids.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 8 images

Recommended textbooks for you


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University