A very young child is prescribed 2.0 ml of an analgesic suspension three times a day for 4/7. How many millilitres of suspension should the pharmacist dispense? О 30 O 24 О 20 O 28 A prescription requires a patient to receive a quantity of indigestion mixture with a dosage regimen of 15 ml four times a day for 5 days. Calculate the total volume that should be supplied to the patient. O 200 ml O 300 ml O 150 ml 100 ml
A very young child is prescribed 2.0 ml of an analgesic suspension three times a day for 4/7. How many millilitres of suspension should the pharmacist dispense? О 30 O 24 О 20 O 28 A prescription requires a patient to receive a quantity of indigestion mixture with a dosage regimen of 15 ml four times a day for 5 days. Calculate the total volume that should be supplied to the patient. O 200 ml O 300 ml O 150 ml 100 ml
Chapter4: Calculations Used In Analytical Chemistry
Section: Chapter Questions
Problem 4.31QAP
Related questions
Question
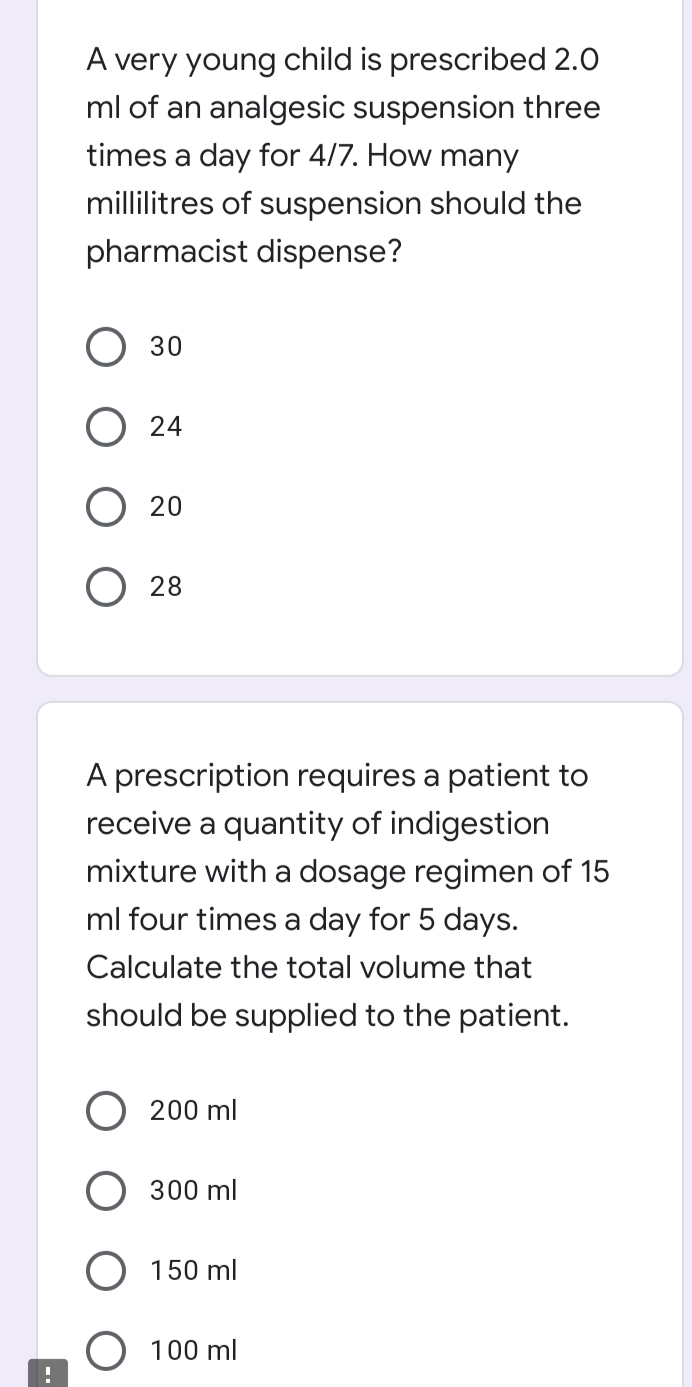
Transcribed Image Text:A very young child is prescribed 2.0
ml of an analgesic suspension three
times a day for 4/7. How many
millilitres of suspension should the
pharmacist dispense?
О 30
O 24
20
28
A prescription requires a patient to
receive a quantity of indigestion
mixture with a dosage regimen of 15
ml four times a day for 5 days.
Calculate the total volume that
should be supplied to the patient.
200 ml
300 ml
150 ml
O 100 ml
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning