a. Draw a block flow diagram to represent the process with only the information that has been given so far. b. Determine which species is the limiting reactant. Do not just select one of the species. c. For 30% conversion of the limiting reactant, show (using atomic species balances or extent of reaction) how much H2W and how much WH are produced
We discovered 50 grams of an interesting new chemical, Jerrodwowzium "JW3" on the lab bench. This chemical is not very well characterized; however, one of your fellow chemical engineering classmates was able to determine that this chemical has a molecular weight of 62. In order to clean this chemical from the lab bench 100 grams of another newly discovered chemical, Laughawazzana, LH3WH (MW 32) was added. Help me
describe this process to another engineer.
(Governing Reaction in Image)
a. Draw a block flow diagram to represent the process with only the information that has been given so far.
b. Determine which species is the limiting reactant. Do not just select one of the species.
c. For 30% conversion of the limiting reactant, show (using atomic species balances or extent of reaction) how much H2W and how much WH are produced
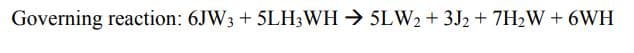
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images









