A. If you are going to prepare Cu(1) solution that will have a 0.2 absorbance when complexed with 1,10- phenanthroline, what will be the Molarity of this Cu(I) solution if this is the limiting reagent? Pls show your step by step solution B. What type of ligand is 1. 10 phenantroline? C. What is coordination number of Cu+1 in the complex ? Cu(1) molar mass=63.546 1,10-phenanthroline molar mass=180.2. Pls use response template for your final answer in A. B and C
A. If you are going to prepare Cu(1) solution that will have a 0.2 absorbance when complexed with 1,10- phenanthroline, what will be the Molarity of this Cu(I) solution if this is the limiting reagent? Pls show your step by step solution B. What type of ligand is 1. 10 phenantroline? C. What is coordination number of Cu+1 in the complex ? Cu(1) molar mass=63.546 1,10-phenanthroline molar mass=180.2. Pls use response template for your final answer in A. B and C
Chapter12: Spectrochemical Methods
Section: Chapter Questions
Problem 13P
Related questions
Question
I NEED HELP PLEASE ASAP!!! Hoping for complete solutions since I’m having a hard time with this. Pls skip if unsure or not willing to answer the subitems (these are all connected for one item). Please show full solution and I'll give thumbs up. Thank you!
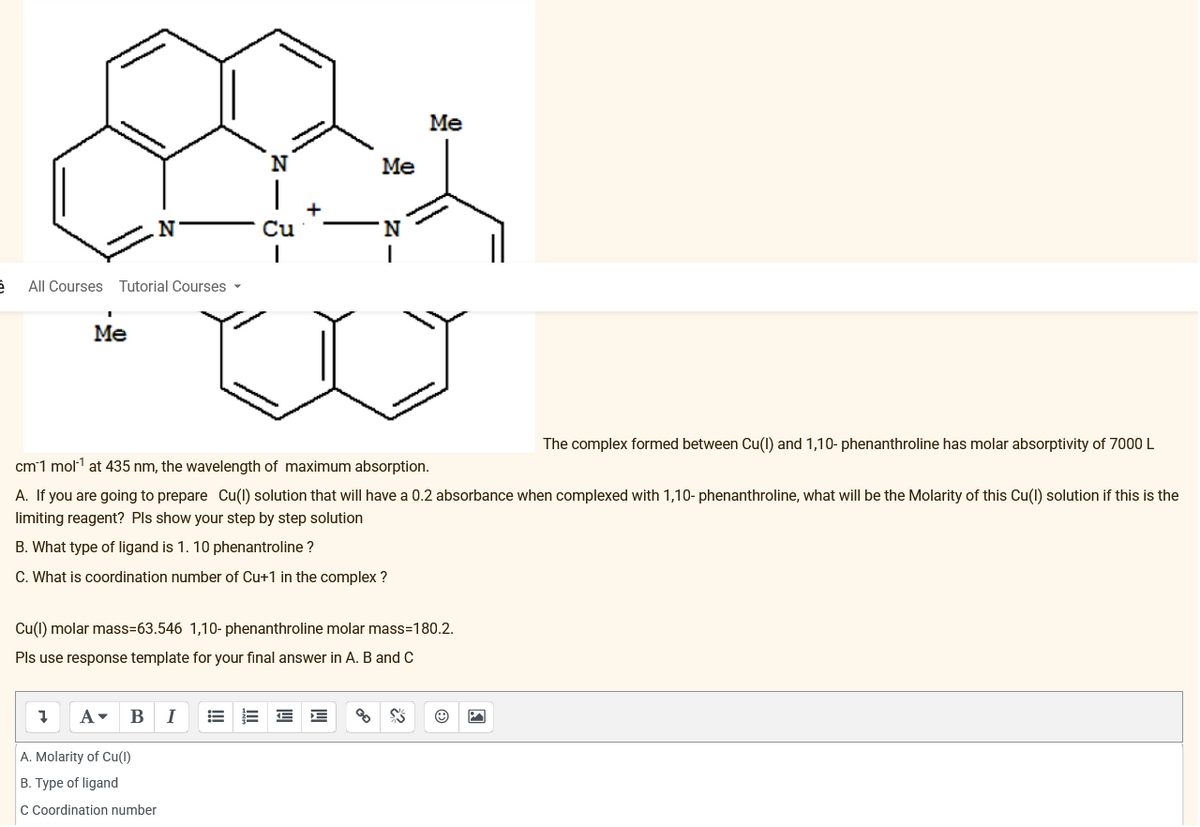
Transcribed Image Text:N
Cu
A. Molarity of Cu(1)
B. Type of ligand
C Coordination number
Me
Me
N
'N
è
All Courses Tutorial Courses ▾
I
Me
The complex formed between Cu(I) and 1,10- phenanthroline has molar absorptivity of 7000 L
cm 1 mol¹ at 435 nm, the wavelength of maximum absorption.
A. If you are going to prepare Cu(I) solution that will have a 0.2 absorbance when complexed with 1,10- phenanthroline, what will be the Molarity of this Cu(I) solution if this is the
limiting reagent? Pls show your step by step solution
B. What type of ligand is 1. 10 phenantroline ?
C. What is coordination number of Cu+1 in the complex?
Cu(1) molar mass-63.546 1,10- phenanthroline molar mass=180.2.
Pls use response template for your final answer in A. B and C
7 A▾ B I
% SS
Ⓒ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
