a. Infrared spectra give information about bonding features and functional groups in molecules O b. Infrared spectra record the transmission of IR radiation O C. Molecular vibrations are due to periodic motions of atoms in molecules, and include bond stretching, torsional changes, and bond angle changes d. Infrared radiation is higher in energy than UV radiation
a. Infrared spectra give information about bonding features and functional groups in molecules O b. Infrared spectra record the transmission of IR radiation O C. Molecular vibrations are due to periodic motions of atoms in molecules, and include bond stretching, torsional changes, and bond angle changes d. Infrared radiation is higher in energy than UV radiation
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter7: Chemical Bonding And Molecular Structure
Section: Chapter Questions
Problem 7.25PAE: 7.25 How does the bond energy of a double bond compare to that of two single bonds between the same...
Related questions
Question
Which of the following statements regarding IR spectroscopy is incorrect?
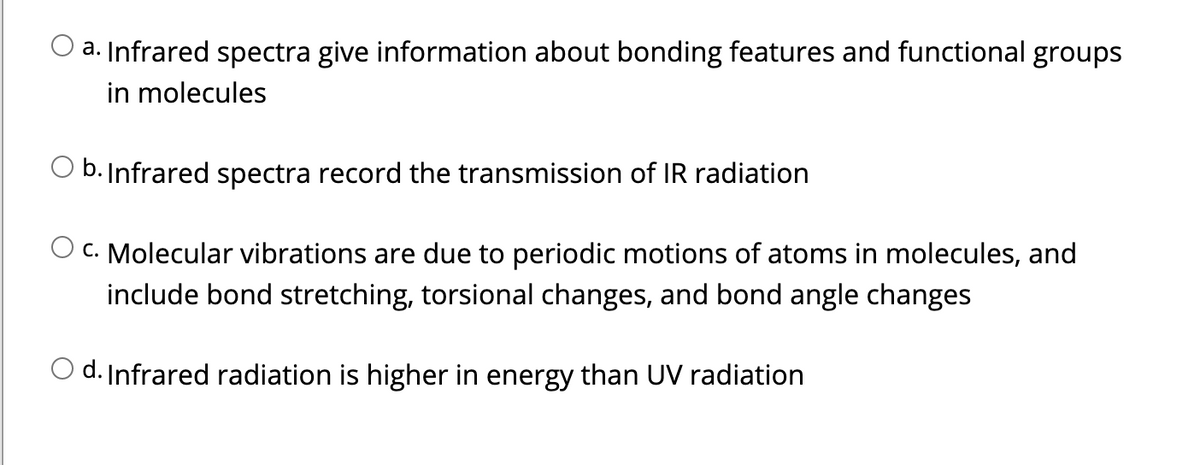
Transcribed Image Text:a. Infrared spectra give information about bonding features and functional groups
in molecules
b. Infrared spectra record the transmission of IR radiation
C. Molecular vibrations are due to periodic motions of atoms in molecules, and
include bond stretching, torsional changes, and bond angle changes
O d. Infrared radiation is higher in energy than UV radiation
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co