a. Nickel has an FCC structure. Given that the atomic radius of Ni is C calculate the number of atoms per square meter on the (100) elemental Ni? Show your calculations.
a. Nickel has an FCC structure. Given that the atomic radius of Ni is C calculate the number of atoms per square meter on the (100) elemental Ni? Show your calculations.
Understanding Motor Controls
4th Edition
ISBN:9781337798686
Author:Stephen L. Herman
Publisher:Stephen L. Herman
Chapter44: Semiconductors
Section: Chapter Questions
Problem 4RQ
Related questions
Question

Transcribed Image Text:b. Given that Na = 6.022x1023 atoms/mol. Calculate the theoretical density of
nickel. Show your calculations.
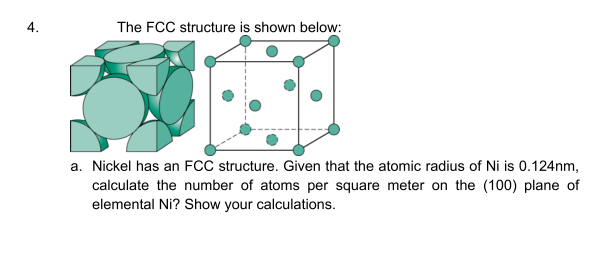
Transcribed Image Text:4.
The FCC structure is shown below:
a. Nickel has an FCC structure. Given that the atomic radius of Ni is 0.124nm,
calculate the number of atoms per square meter on the (100) plane of
elemental Ni? Show your calculations.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Understanding Motor Controls
Mechanical Engineering
ISBN:
9781337798686
Author:
Stephen L. Herman
Publisher:
Delmar Cengage Learning

Understanding Motor Controls
Mechanical Engineering
ISBN:
9781337798686
Author:
Stephen L. Herman
Publisher:
Delmar Cengage Learning