a. Which phases are present? b. What is the complete composition of each of these phases? c. What is the weight amount of each of these phases?
a. Which phases are present? b. What is the complete composition of each of these phases? c. What is the weight amount of each of these phases?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter13: The Chemistry Of Solutes And Solutions
Section: Chapter Questions
Problem 29QRT
Related questions
Question
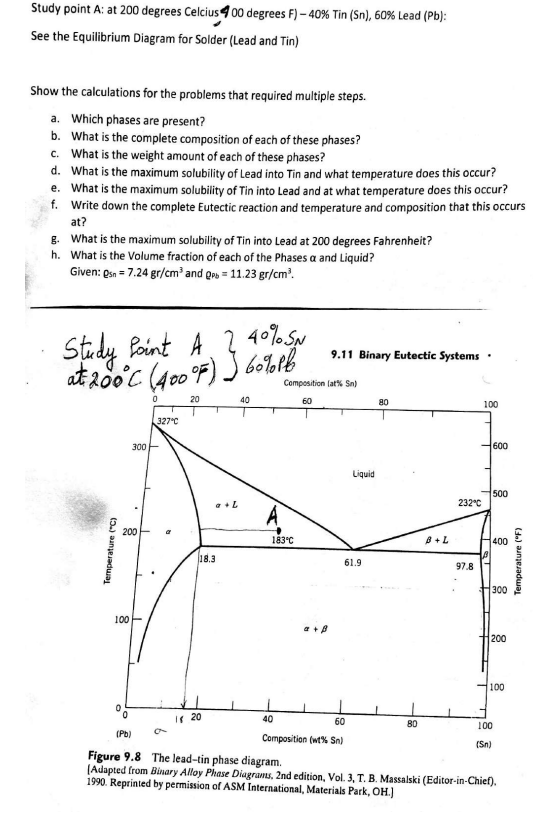
Transcribed Image Text:Study point A: at 200 degrees Celcius 400 degrees F) -40% Tin (Sn), 60% Lead (Pb):
See the Equilibrium Diagram for Solder (Lead and Tin)
Show the calculations for the problems that required multiple steps.
a. Which phases are present?
b.
What is the complete composition of each of these phases?
c. What is the weight amount of each of these phases?
d. What is the maximum solubility of Lead into Tin and what temperature does this occur?
e. What is the maximum solubility of Tin into Lead and at what temperature does this occur?
f. Write down the complete Eutectic reaction and temperature and composition that this occurs
at?
g. What is the maximum solubility of Tin into Lead at 200 degrees Fahrenheit?
h.
What is the Volume fraction of each of the Phases a and Liquid?
Given: Osn = 7.24 gr/cm³ and Q=11.23 gr/cm³.
2 40% SN
Study Point A
at 200°C (400°F) 60% Pb
20
Temperature (°C)
300
200
100-
(Pb)
0
327°C
18.3
120
40
A
183°C
40
Composition (at% Sn)
60
9.11 Binary Eutectic Systems
a + A
T
Liquid
61.9
60
Composition (wt% Sn)
80
80
B+L
232°C
97.8
100
21
I
T
600
500
400
300
200
100
100
(Sn)
Figure 9.8 The lead-tin phase diagram.
(Adapted from Binary Alloy Phase Diagrams, 2nd edition, Vol. 3, T. B. Massalski (Editor-in-Chief).
1990. Reprinted by permission of ASM International, Materials Park, OH.)
Temperature (°F)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
at 100C what is the maximum solubility (a) of Pb in Sn and (b) of Sn in Pb? The lead-tin phase diagram is shown in the animated figure
wt% Pb ?
wt% Sn ?
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning