A0.0545 kg chunk of an unknown metal that has been in boiling water for several minutes is quickly dropped into an insulating Styrofoam beaker that contains 0.462 kg of water at room temperature (20.0° C). After waiting for a few minutes, you observe that the water's temperature has reached a constant value of 22.0° C. Part A Assuming that the Styrofoam absorbs a negligibly small amount of heat and that no heat was lost to the surroundings, what is the specific heat of the metal? Express your answer in joules per kilogram-kelvin to three significant figures. ? C = J/(kg K) Submit Request Answer Part B Use the table below to identify the metal. Specific heat (c) Material J/(kg K) cal/(g K) Solids Lead 0.13 x 10 0.031 Mercury 0.14 x 103 0.033 Silver 0.23 x 10 0.056 Copper 0.39 x 10 0.093 Iron 0.47 x 10 0.112 Marble (CaCO3) 0.88 x 103 0.21 Salt 0.88 x 10 0.21 0.91 x 10 0.217 Aluminum Bervllium 1.97 y 10 0.471
A0.0545 kg chunk of an unknown metal that has been in boiling water for several minutes is quickly dropped into an insulating Styrofoam beaker that contains 0.462 kg of water at room temperature (20.0° C). After waiting for a few minutes, you observe that the water's temperature has reached a constant value of 22.0° C. Part A Assuming that the Styrofoam absorbs a negligibly small amount of heat and that no heat was lost to the surroundings, what is the specific heat of the metal? Express your answer in joules per kilogram-kelvin to three significant figures. ? C = J/(kg K) Submit Request Answer Part B Use the table below to identify the metal. Specific heat (c) Material J/(kg K) cal/(g K) Solids Lead 0.13 x 10 0.031 Mercury 0.14 x 103 0.033 Silver 0.23 x 10 0.056 Copper 0.39 x 10 0.093 Iron 0.47 x 10 0.112 Marble (CaCO3) 0.88 x 103 0.21 Salt 0.88 x 10 0.21 0.91 x 10 0.217 Aluminum Bervllium 1.97 y 10 0.471
Chapter1: Temperature And Heat
Section: Chapter Questions
Problem 80P: How many grams of coffee must evaporate from 350 g of coffee in a 100-g glass cup to cool the coffee...
Related questions
Question
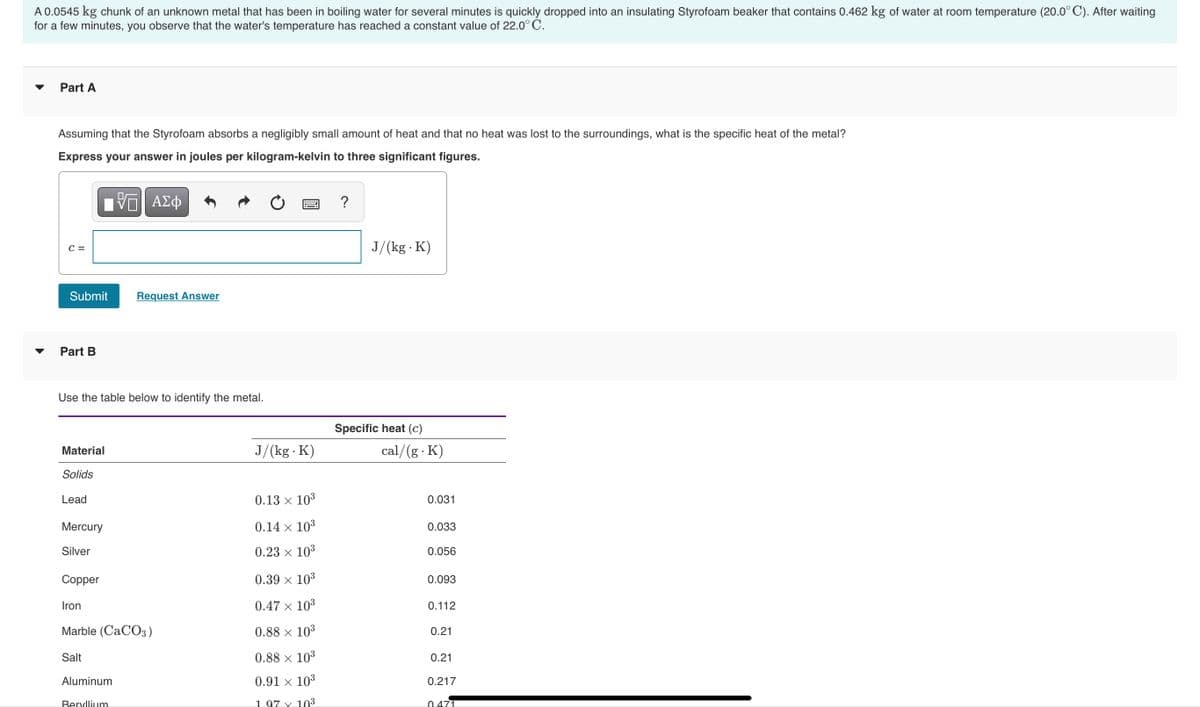
Transcribed Image Text:A 0.0545 kg chunk of an unknown metal that has been in boiling water for several minutes is quickly dropped into an insulating Styrofoam beaker that contains 0.462 kg of water at room temperature (20.0° C). After waiting
for a few minutes, you observe that the water's temperature has reached a constant value of 22.0° C.
Part A
Assuming that the Styrofoam absorbs a negligibly small amount of heat and that no heat was lost to the surroundings, what is the specific heat of the metal?
Express your answer in joules per kilogram-kelvin to three significant figures.
ΑΣφ
J/(kg · K)
C =
Submit
Request Answer
Part B
Use the table below to identify the metal.
Specific heat (c)
Material
J/(kg K)
cal/(g K)
Solids
Lead
0.13 x 103
0.031
Mercury
0.14 × 103
0.033
Silver
0.23 × 103
0.056
Copper
0.39 × 10³
0.093
Iron
0.47 x 103
0.112
Marble (CACO3)
0.88 × 10³
0.21
Salt
0.88 × 103
0.21
Aluminum
0.91 × 103
0.217
Beryllium
1 97 x 103
0.471
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning